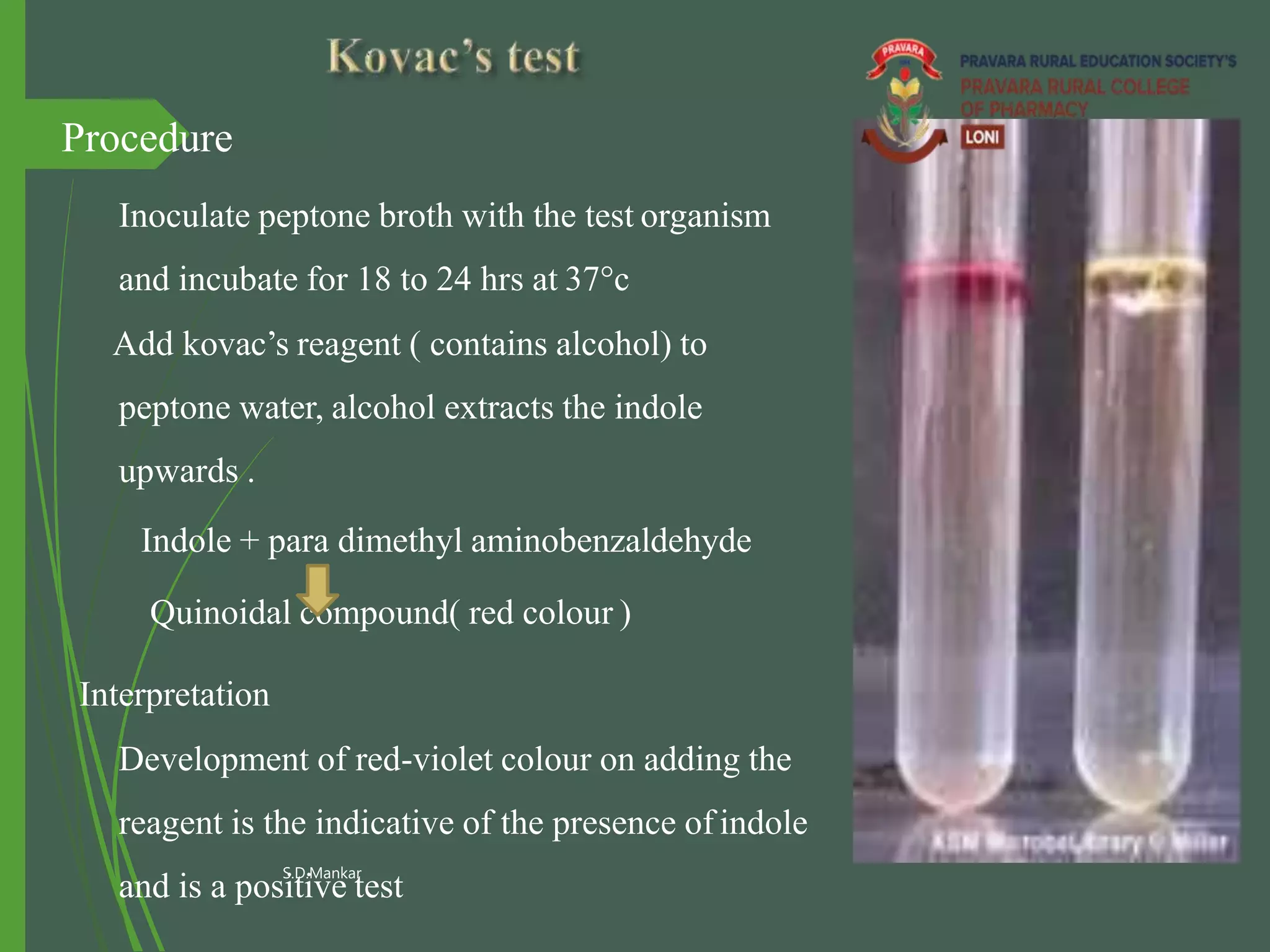
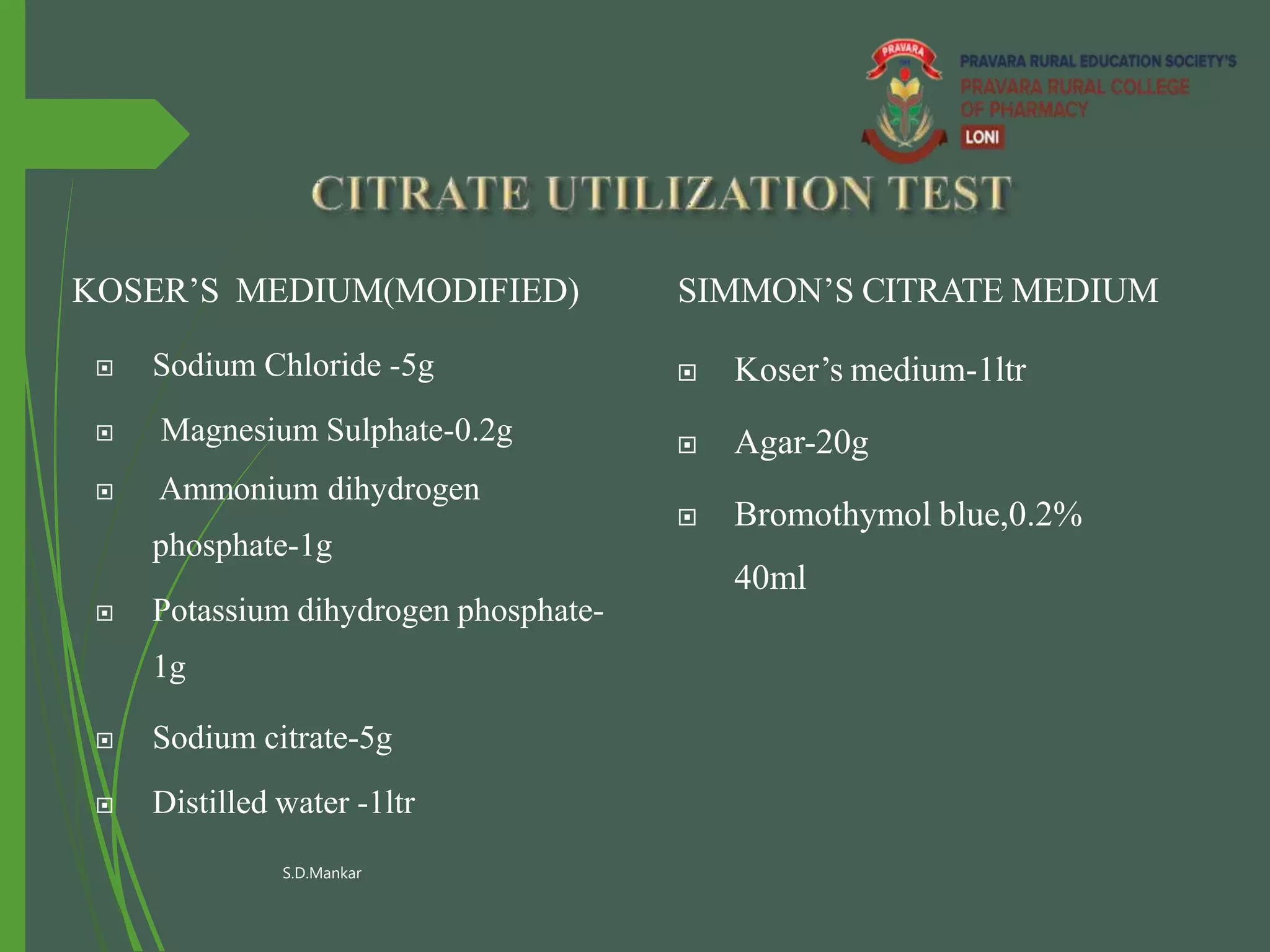
This document describes the citrate utilization test procedure and principles. The citrate utilization test is used to determine if an organism can use sodium citrate as its sole carbon source. The test medium contains sodium citrate as the only carbon source and ammonium salts as the sole nitrogen source. If the organism can metabolize the citrate, it will alkalinize the medium which is indicated by a color change of the bromothymol blue indicator from green to blue. Positive results are demonstrated by a color change to deep blue within 24-48 hours.