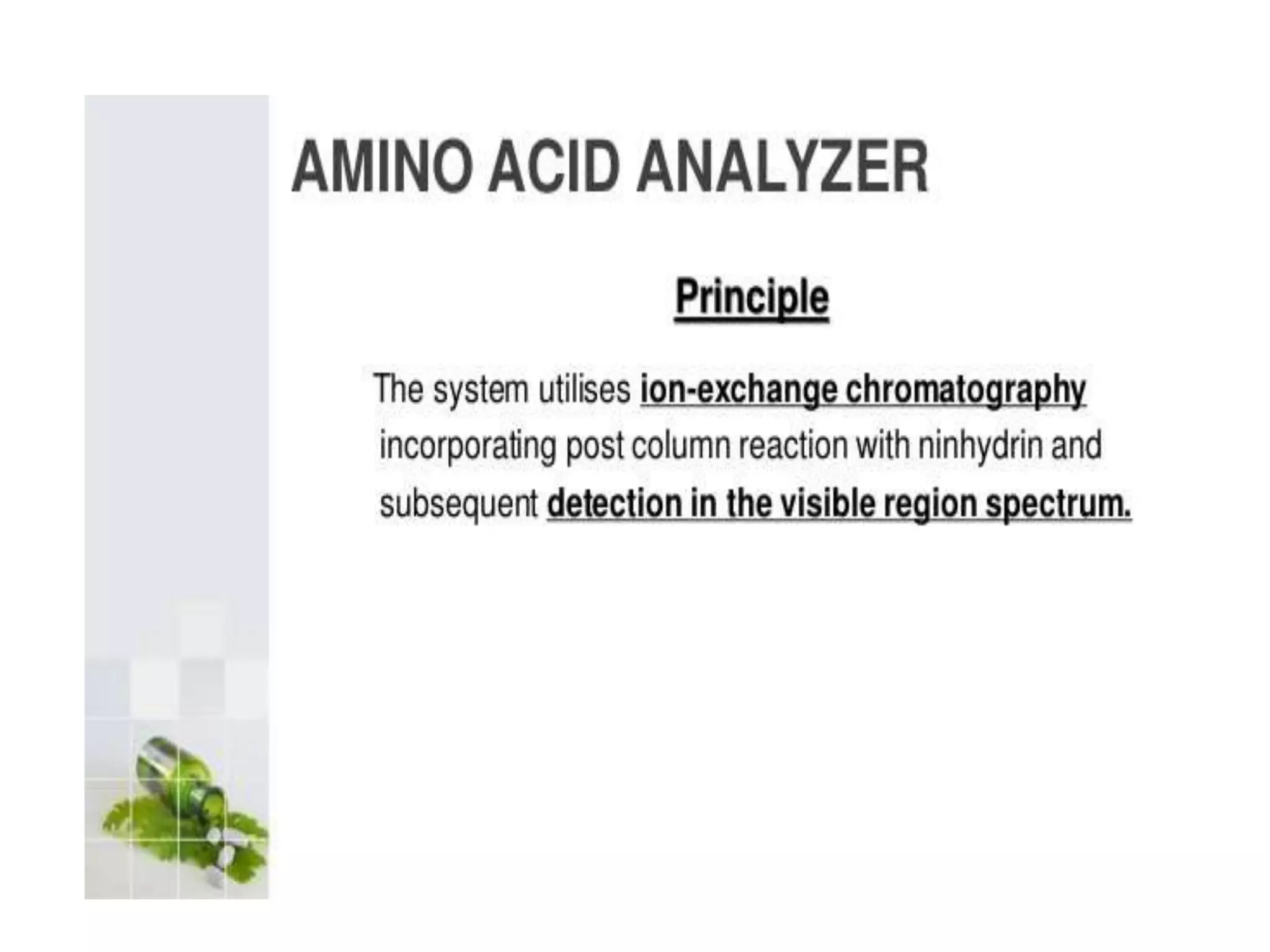
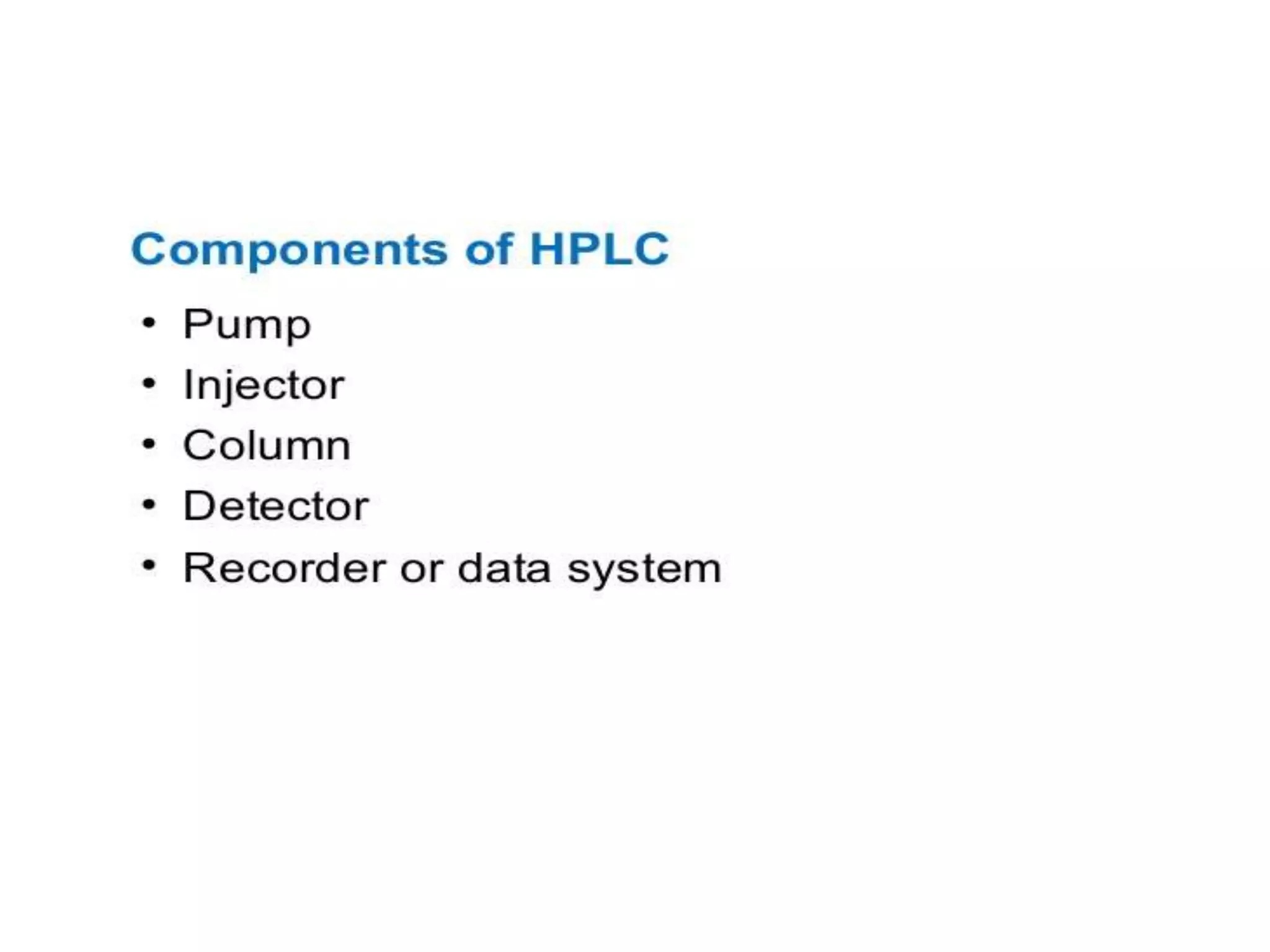
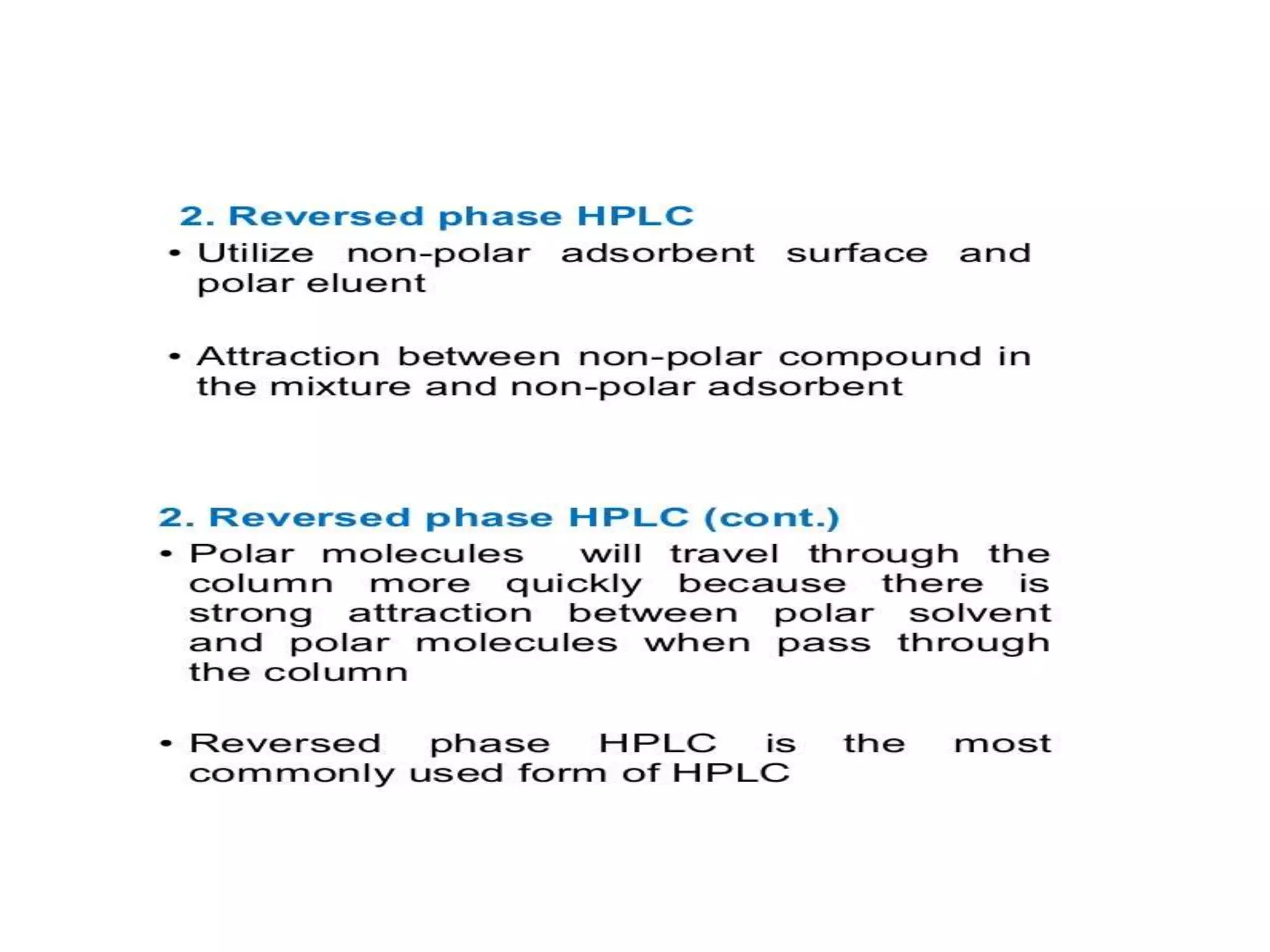
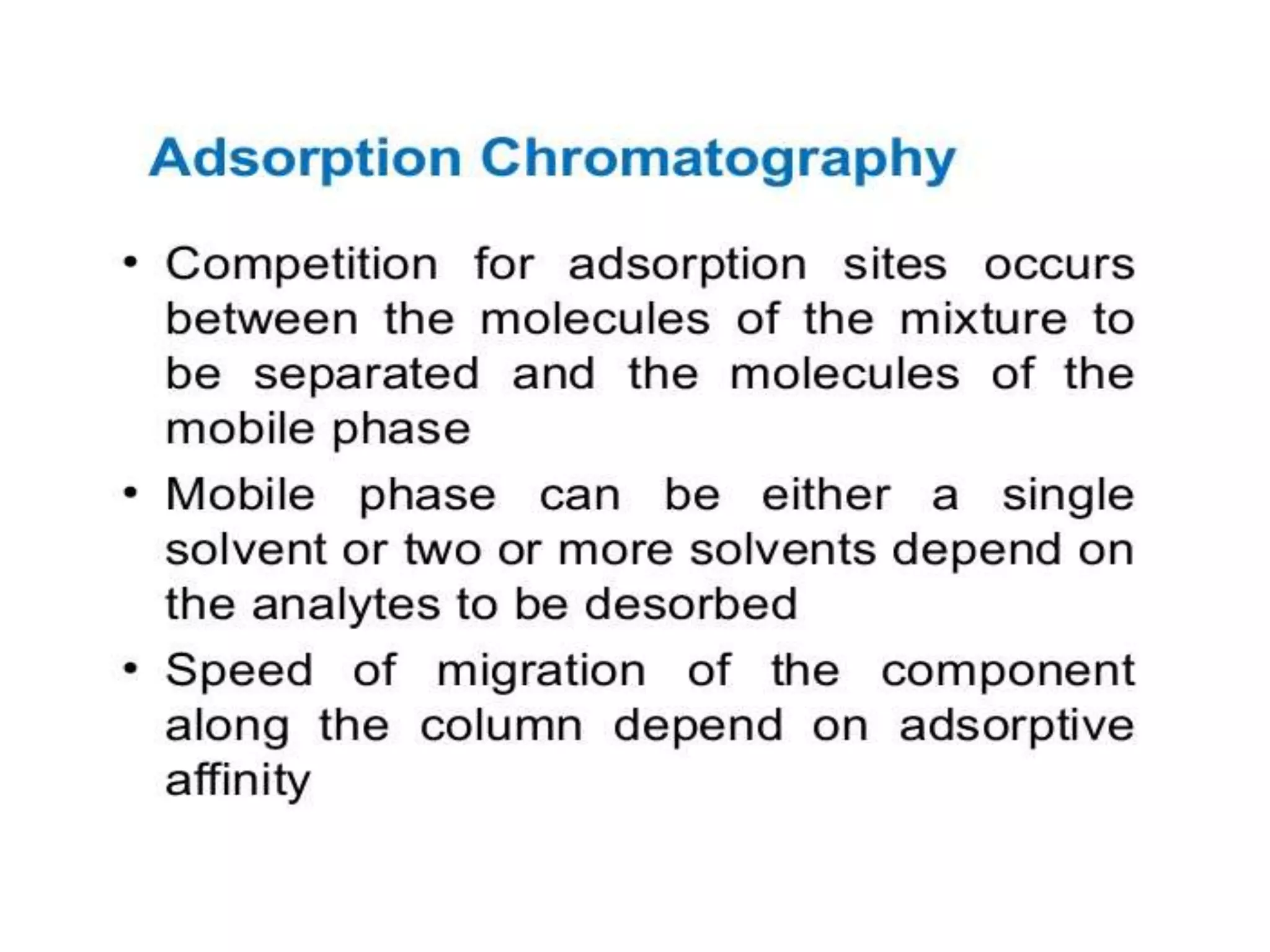
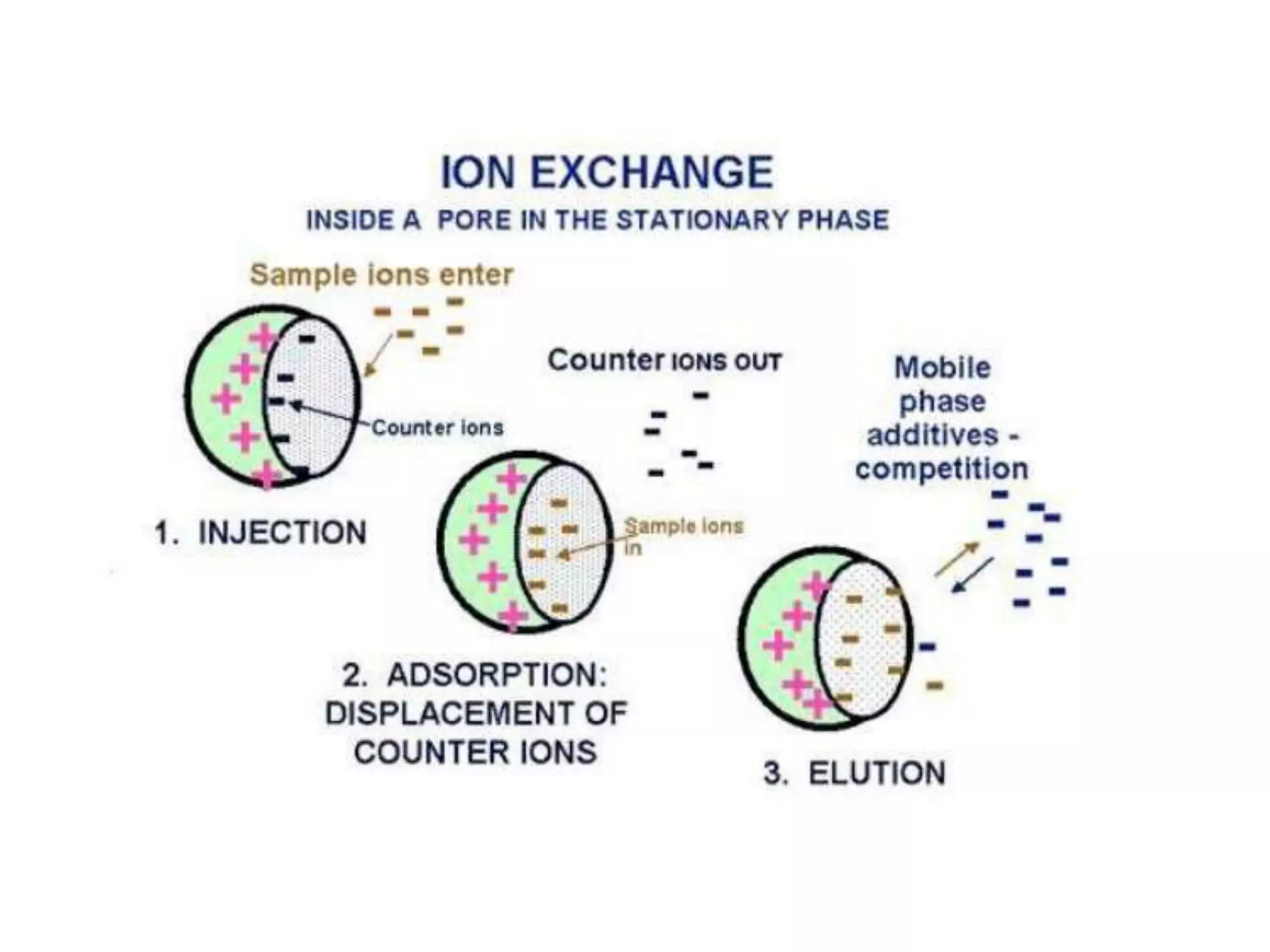
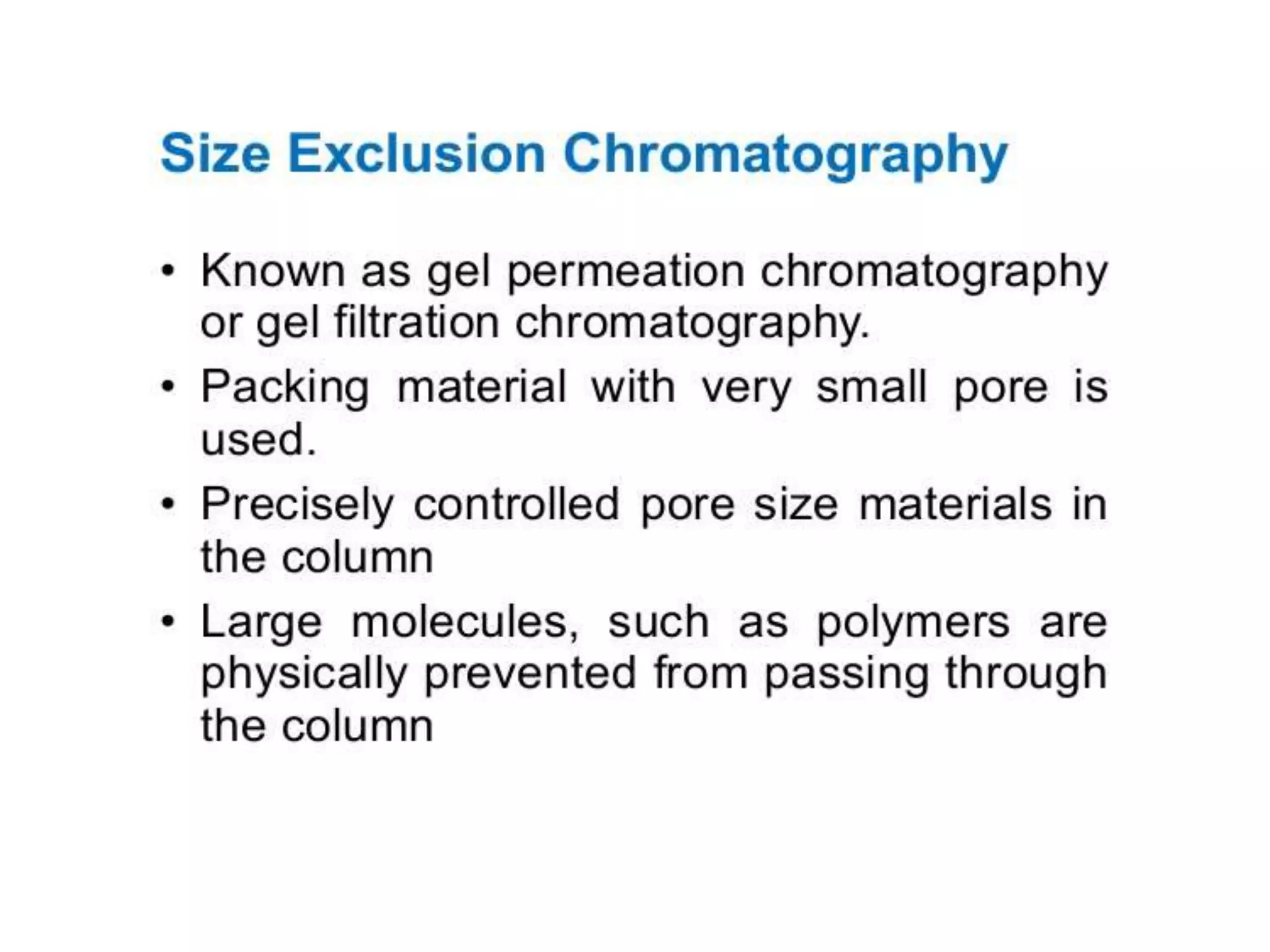
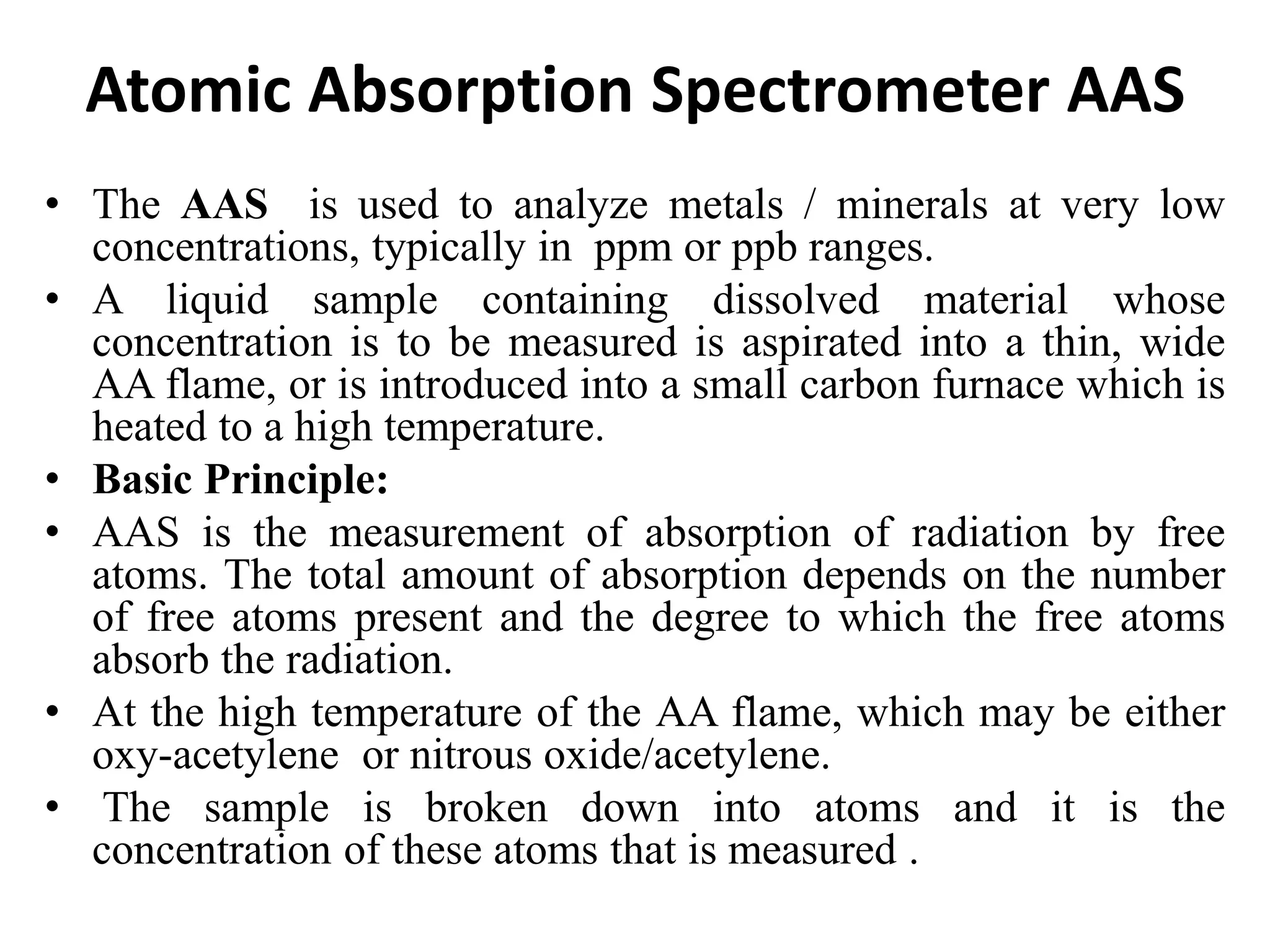
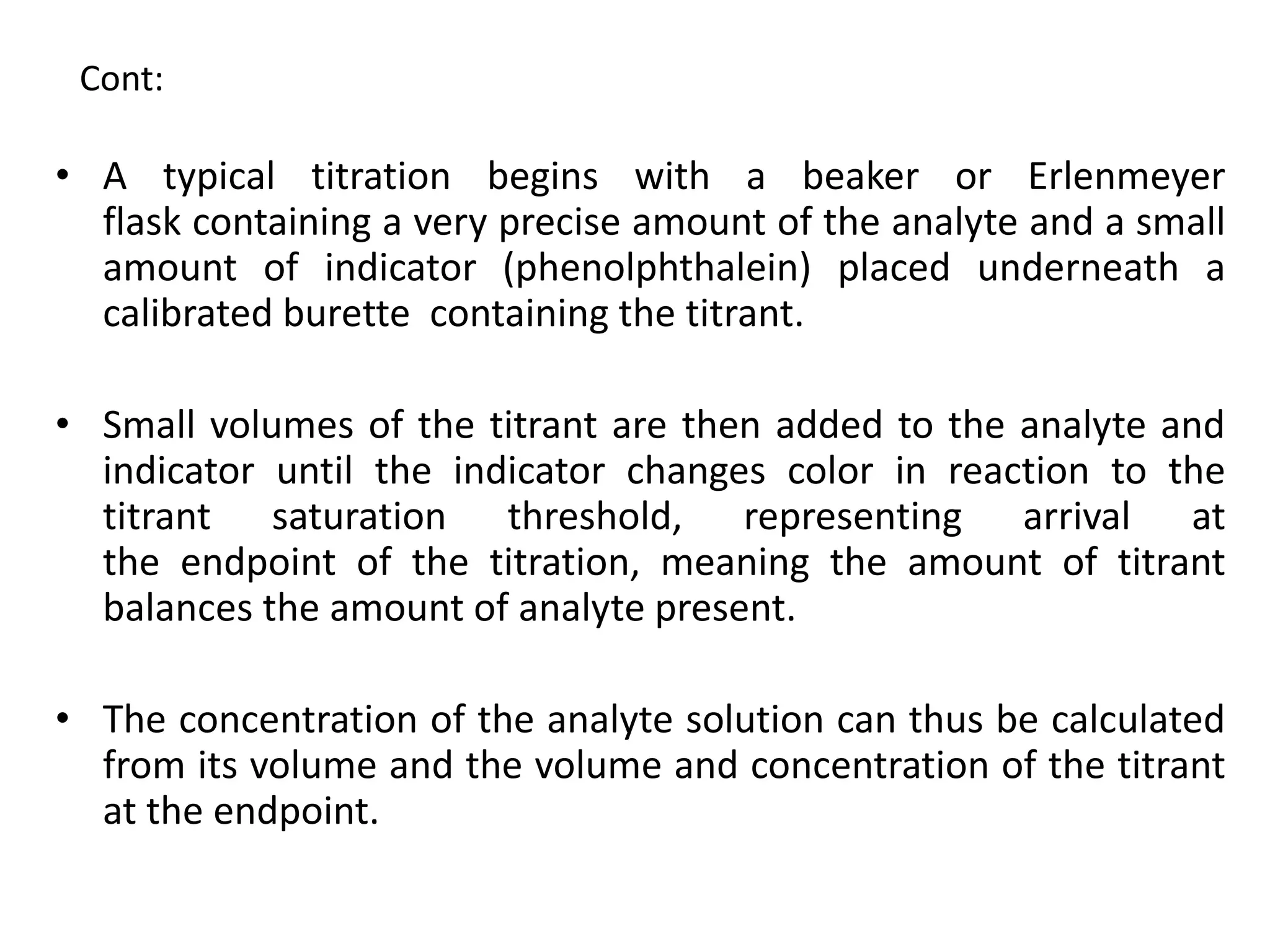
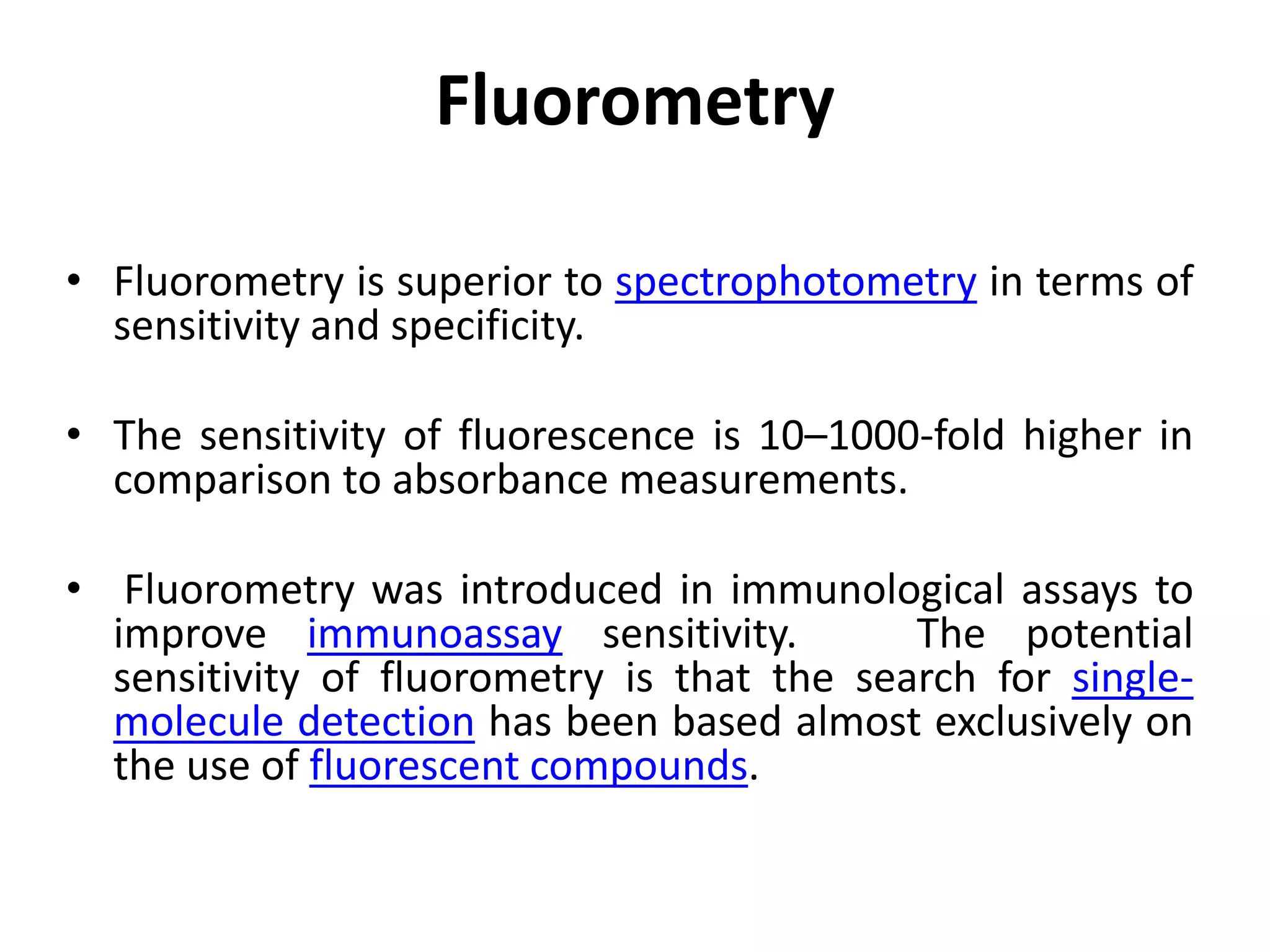
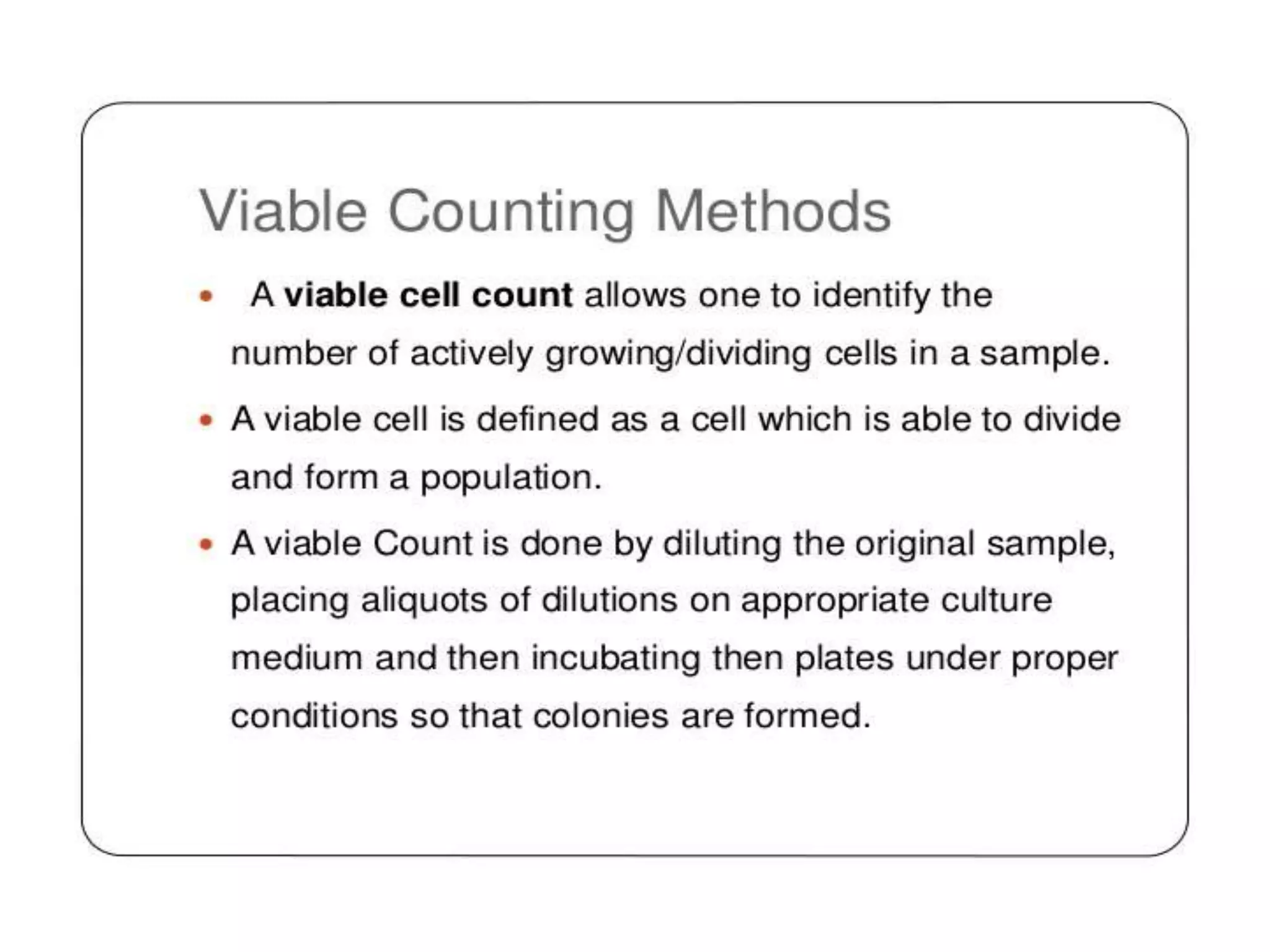
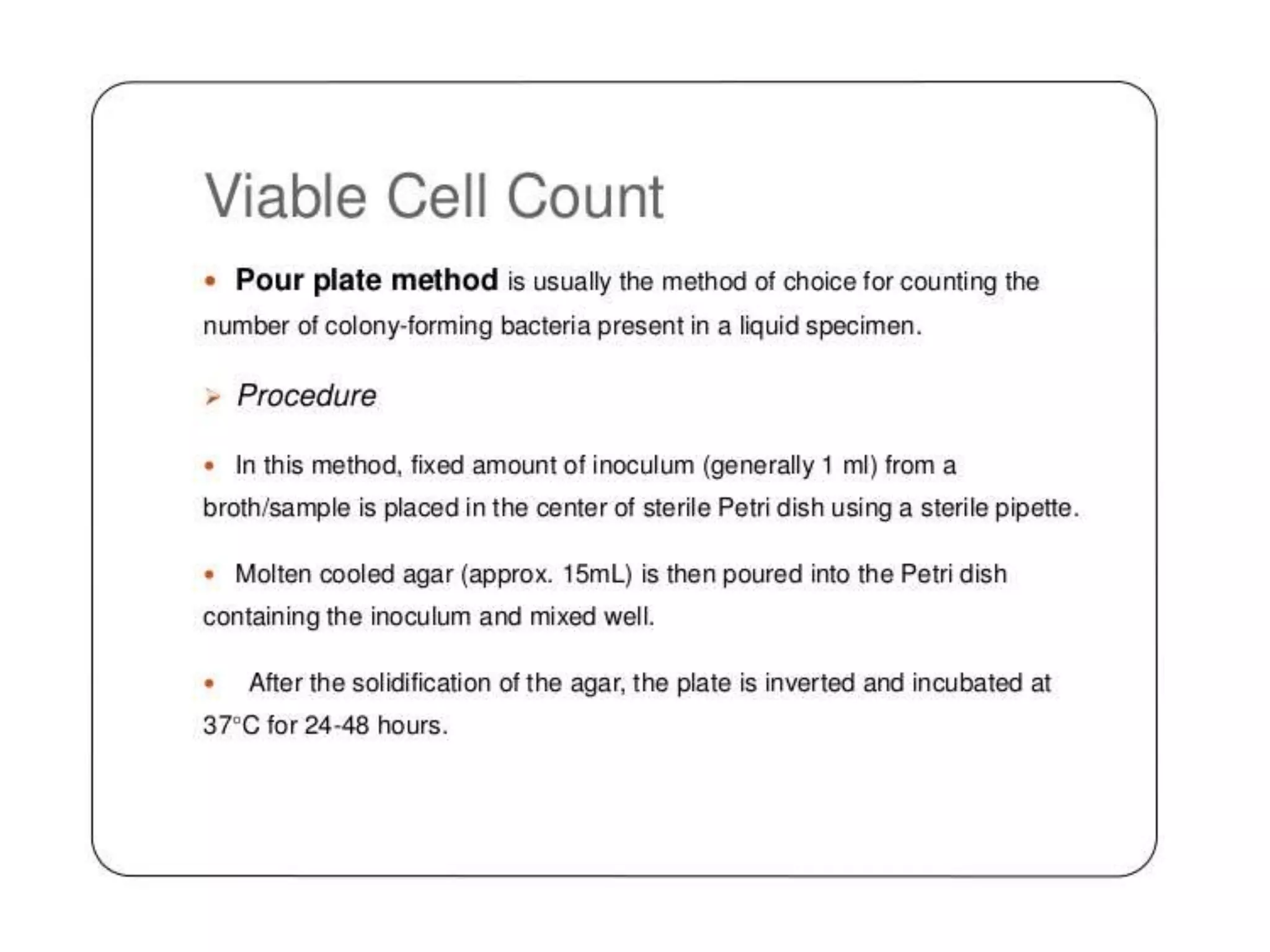
The document describes several different analytic techniques used in chemistry and biochemistry, including amino acid analysis, spectrophotometry, atomic absorption spectrometry, titrimetry, gravity separation, polarimetry, and fluorometry. Amino acid analysis uses ion exchange liquid chromatography to separate and quantify amino acids. Spectrophotometry measures light absorption to determine chemical concentrations. Atomic absorption spectrometry analyzes metals using flame or furnace atomic absorption. Titrimetry determines concentrations via acid-base or redox reactions with a standard solution. Gravity separation separates components by specific weight. Polarimetry measures sample rotation of polarized light. Fluorometry has greater sensitivity than spectrophotometry in detecting fluorescent compounds.