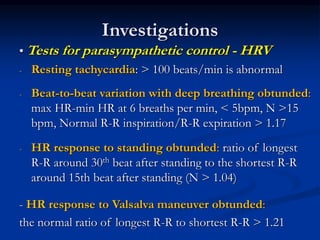
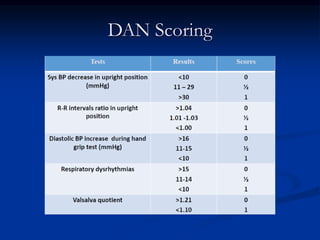
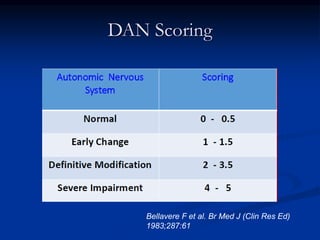
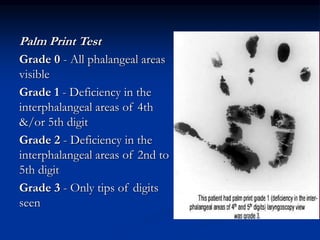
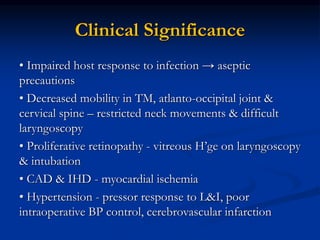
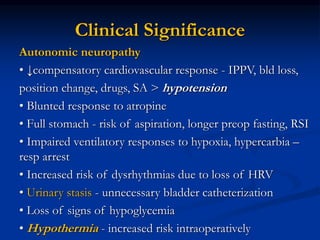
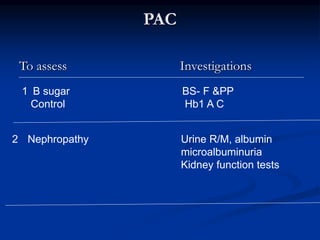
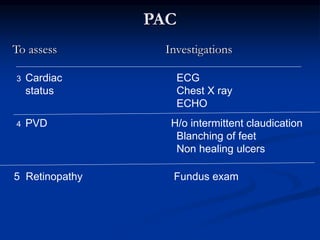
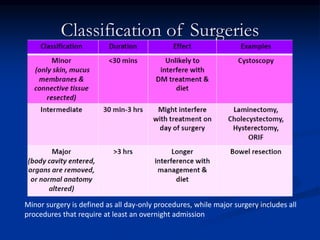
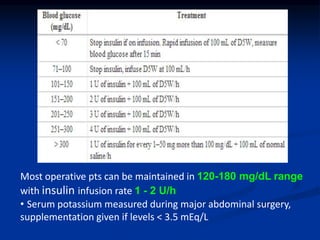
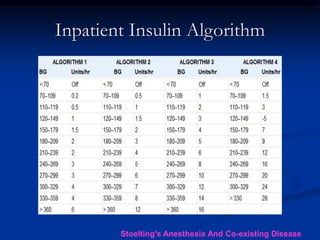
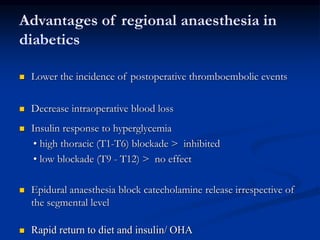
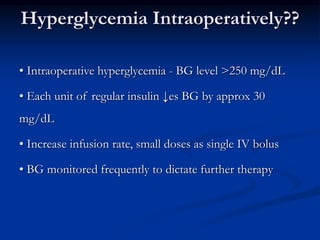
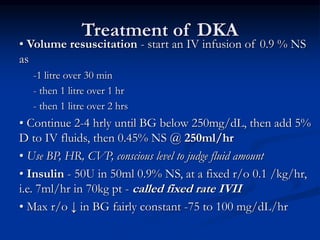
This document presents a case study of a 56-year-old man with type 2 diabetes presenting with a non-healing ulcer on his right foot following toe amputation. It provides details of his medical history, examination findings, lab investigations, and discusses diabetic foot ulcers and complications. The key points are:
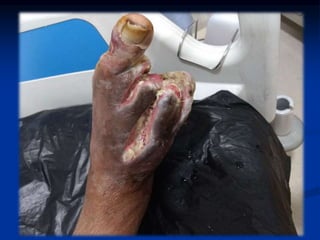
1) The patient had type 2 diabetes for 10 years and was non-compliant with medication, presenting with a non-healing ulcer on his right foot post amputation of toes.
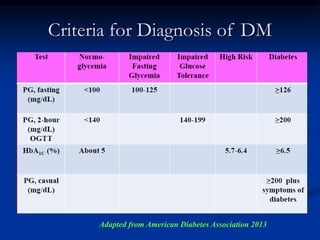
2) Examination found an irregular ulcer on his right foot with signs of infection. Investigations showed renal dysfunction and hyperglycemia.
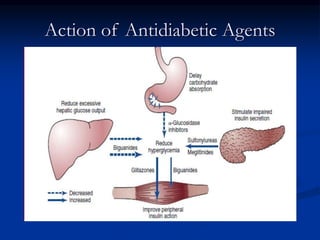
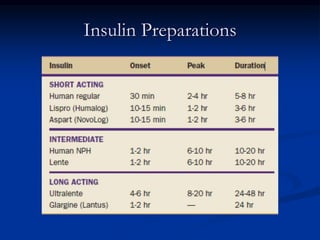
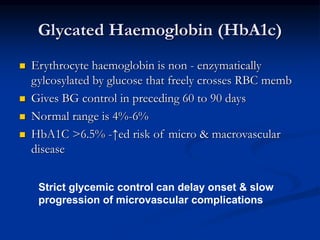
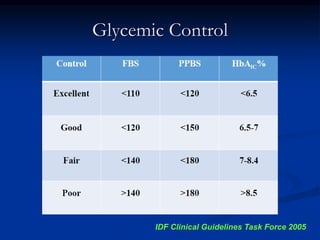
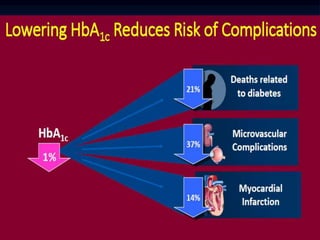
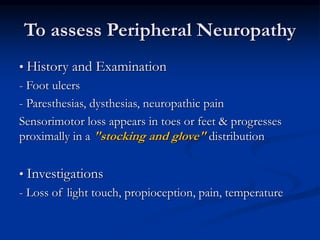
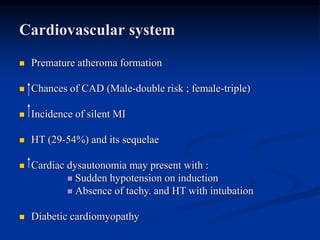
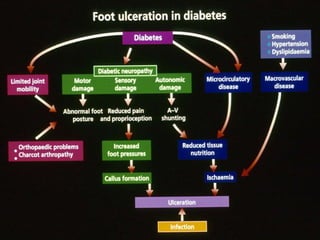
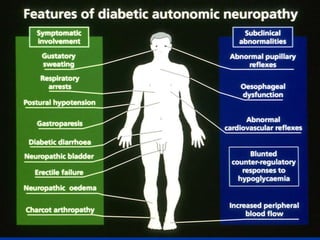
3) Diabetic foot ulcers are a major complication