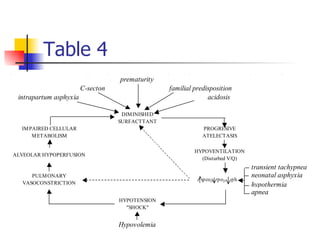
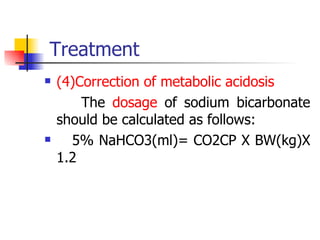
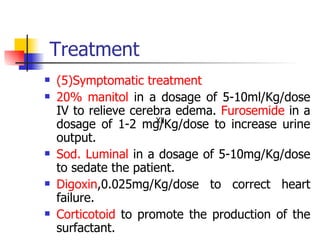
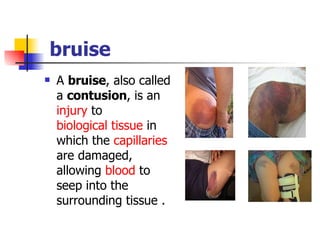
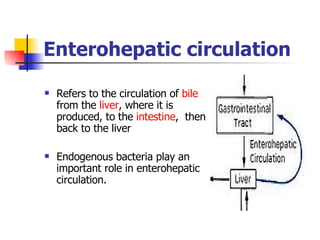
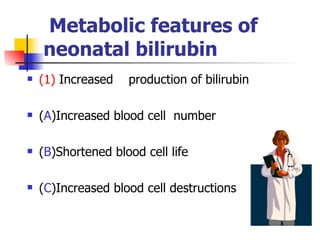
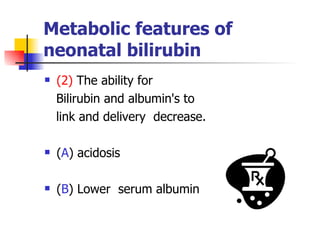
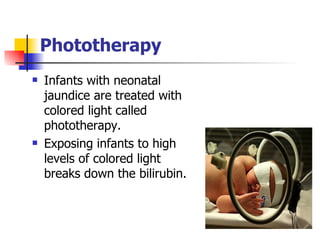
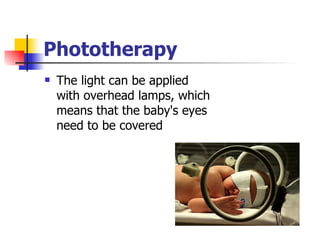
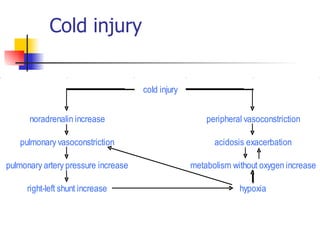
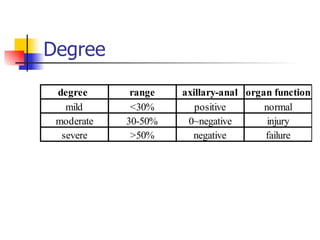
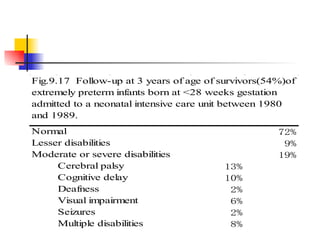
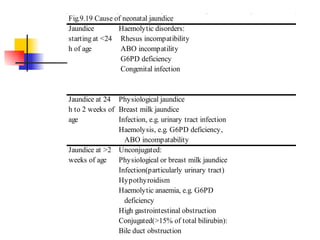
Neonatal respiratory distress syndrome (NRDS), also known as hyaline membrane disease, is a condition caused by insufficient pulmonary surfactant production in premature infants. It affects gas exchange in the lungs and can be life-threatening. The diagnosis is based on clinical presentation, chest x-rays, and blood gas analysis showing hypoxemia and respiratory acidosis. Treatment focuses on supportive care including oxygen supplementation and mechanical ventilation if needed. Prevention centers around reducing preterm birth through good prenatal care and administering corticosteroids to speed up lung maturation.