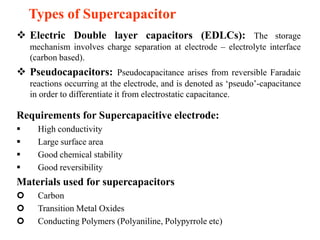
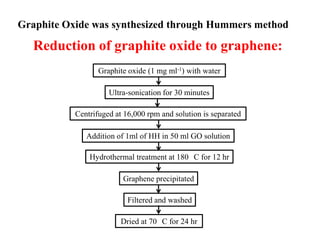
This document summarizes a study on developing a graphene-based coin cell symmetric supercapacitor. Graphene thin films were synthesized through a hydrothermal reduction of graphite oxide and then screen printed onto current collectors. The graphene films were characterized and shown to have a high surface area. A coin cell device was fabricated using the graphene electrodes and tested electrochemically. The device demonstrated a high specific capacitance of 81 F/g, specific energy of 24.5 Wh/kg, and specific power of 10 kW/kg, showing promise for portable electronic applications. Further improvements could be made through composites of graphene with metals or polymers.