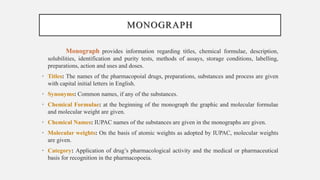
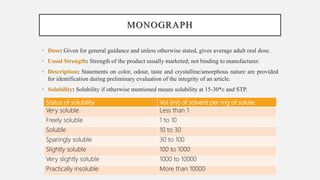
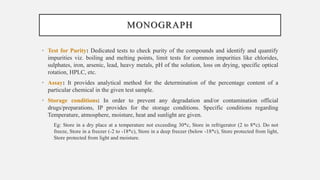
The document discusses pharmacopoeias, which are compendiums published under government authority that provide drug standards. It notes the origins of the term from Greek and provides examples like the Indian, British, and United States Pharmacopoeias. The Indian Pharmacopoeia is discussed in detail, including its history from early publications to the current 8th edition. Key aspects of pharmacopoeias like their role in quality control and international harmonization of standards are mentioned. The format and typical sections of pharmacopoeial monographs are outlined.