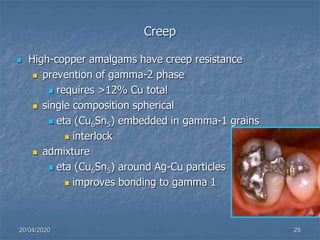
The document outlines the properties and characteristics of dental amalgam, including its strength, dimensional changes, corrosion resistance, and biological effects. Key factors influencing these properties include the composition of the alloys, manipulation techniques, and the presence of specific phases such as gamma-1 and gamma-2. Additionally, it addresses the creep behavior, thermal properties, and implications for dental practices and patient health.