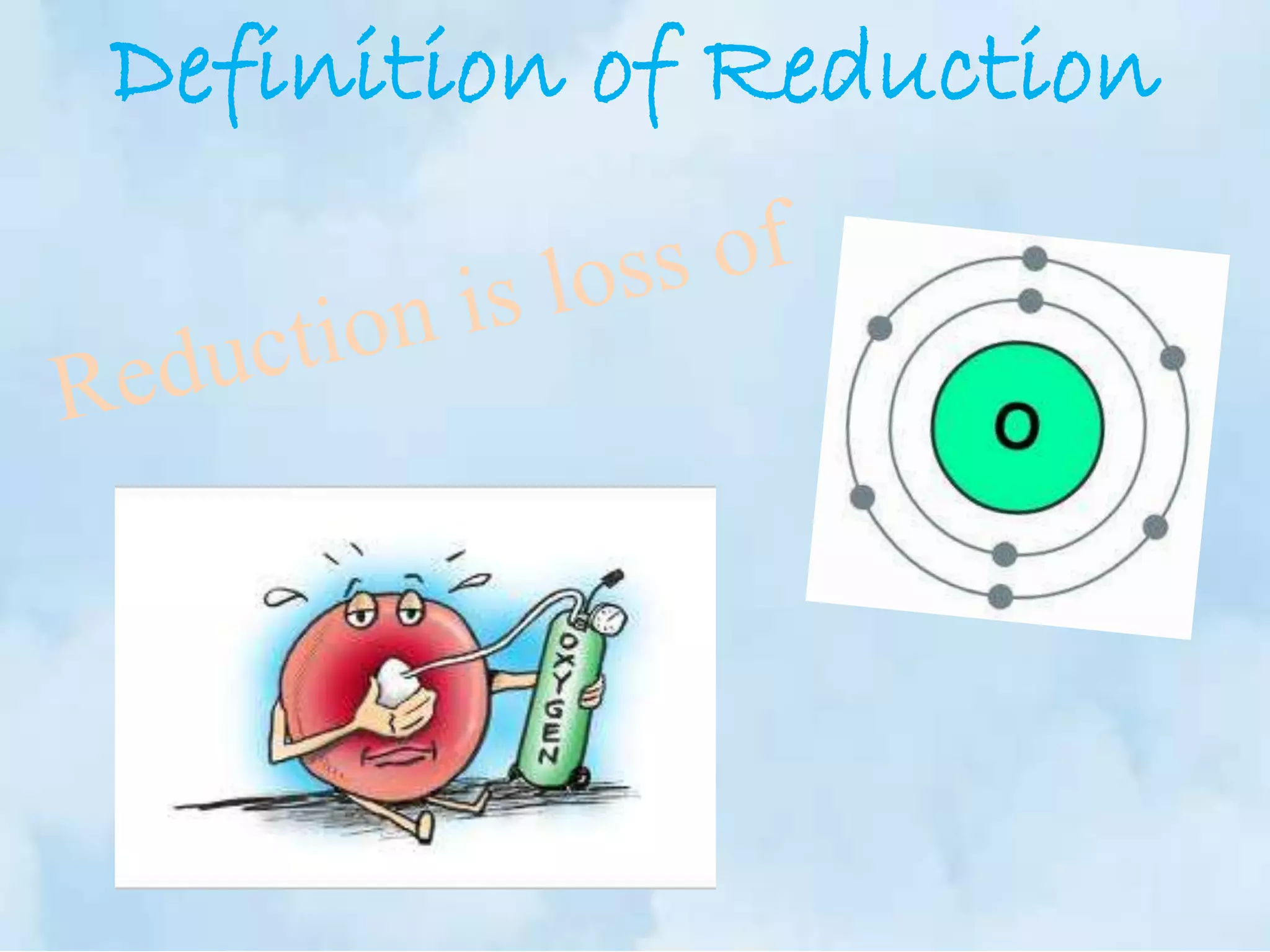
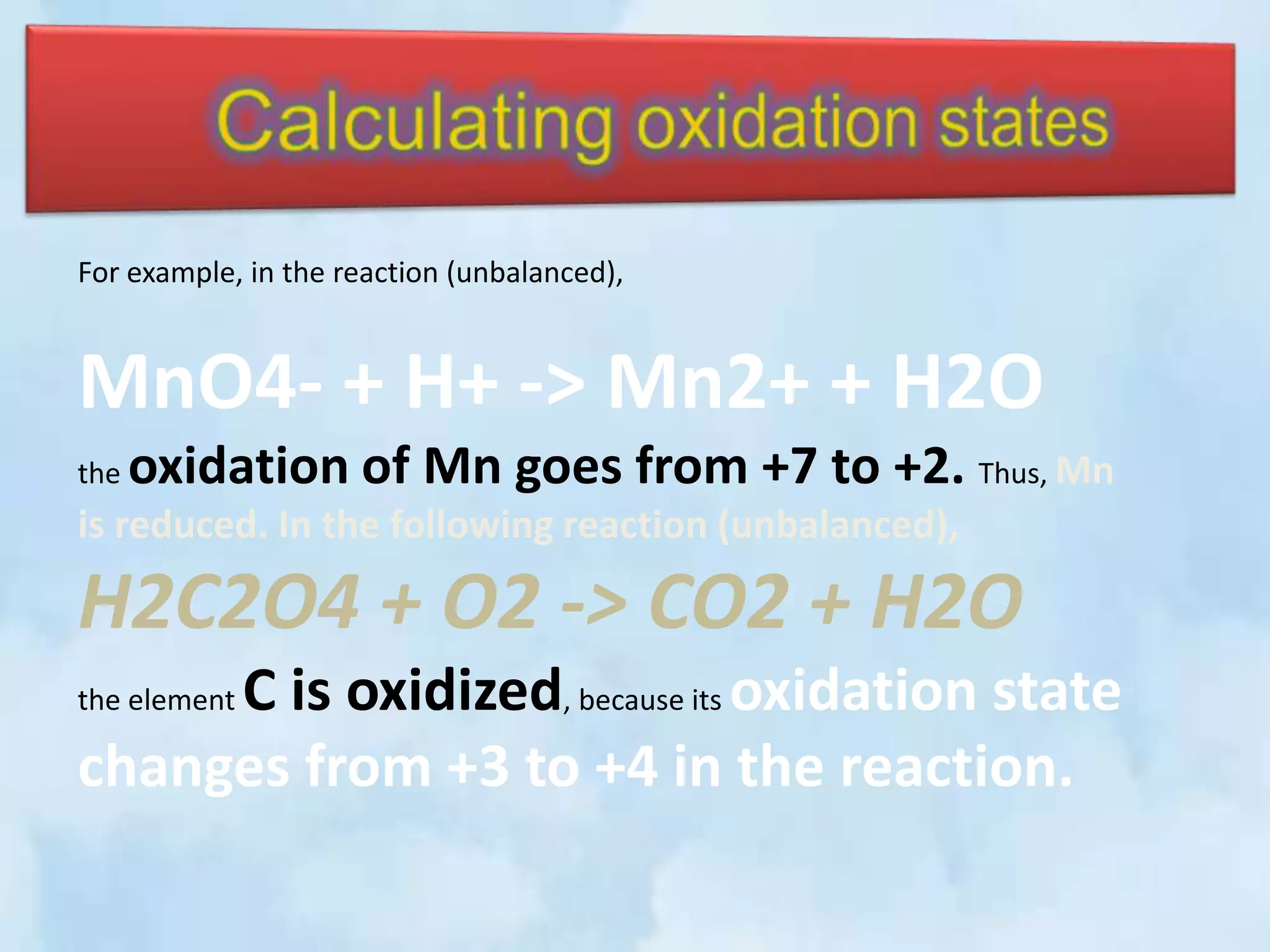
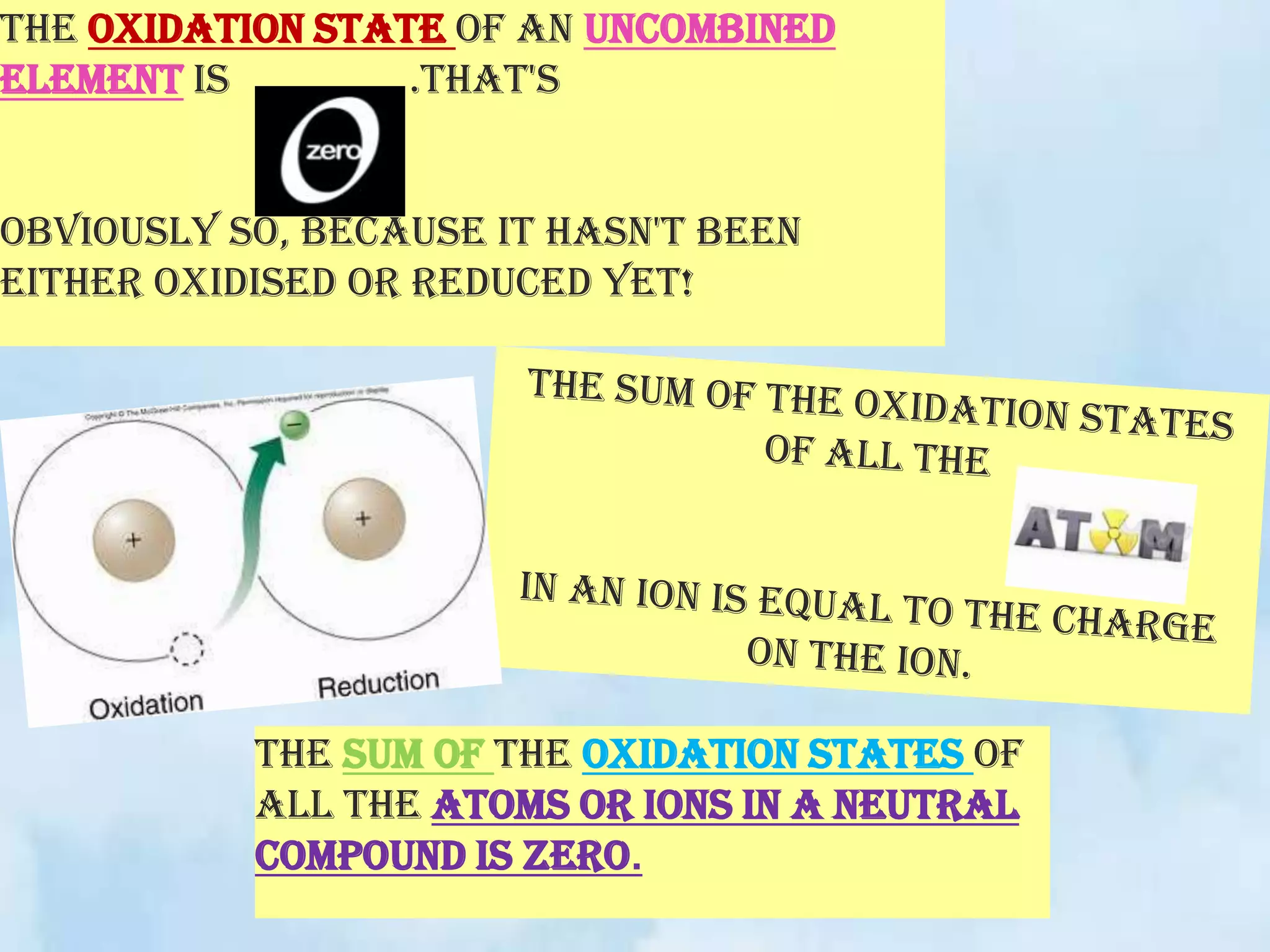
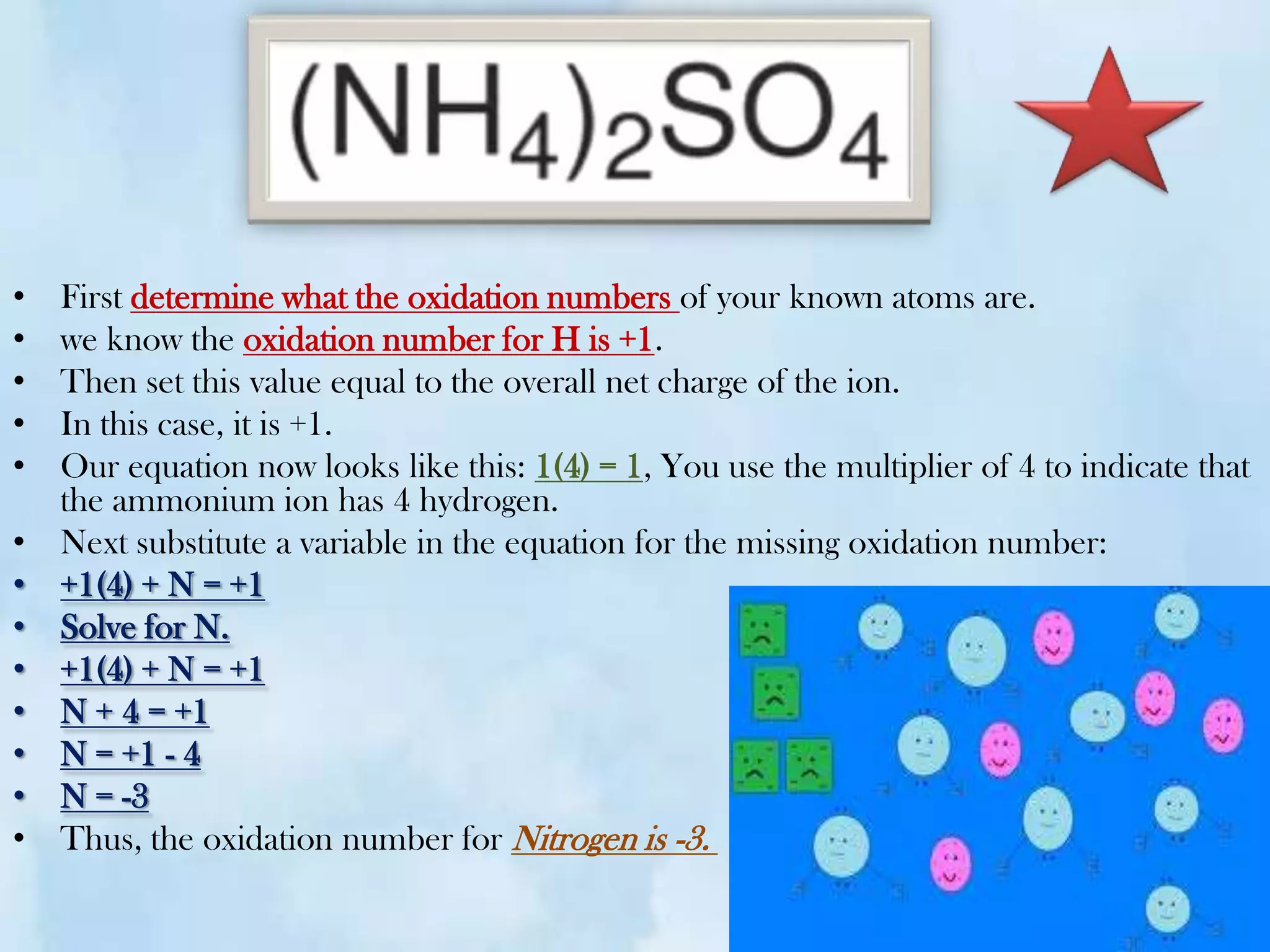
The document explains the concepts of oxidation and reduction, emphasizing that oxidation involves the loss of electrons while reduction involves the gain of electrons. It illustrates these concepts with chemical reactions, detailing changes in oxidation states of manganese and carbon. Additionally, it provides a method for determining oxidation numbers in compounds.