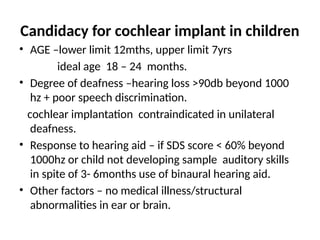
Deaf-mutism," or more accurately, "congenital deafness with mutism," refers to individuals born with both deafness and an inability to speak, often stemming from underlying genetic or developmental factors.
Here's a more detailed explanation:
Definition:
"Deaf-mutism" describes a condition where individuals are congenitally deaf and also have an inability to speak.











![• The growing network of connections in the
developing child enables the child to make
sense of the sounds [complex-language].
• This network develops best in the child as the
plasticity of the brain is maximum in the very
early years of age.
• Early identification of deafness and earliest
possible institution of remedial measures in
child is the remedy in congenital hearing loss.](https://image.slidesharecdn.com/deafmutism-250410181703-7e332506/85/DEAF-MUTISM-power-point-presentation-ppt-16-320.jpg)















![• 5-6 mths
Localisation of sound
• noise makers [rattle drums] 50db
• Soft speech 25db intensity- no response –
higher intensity used.](https://image.slidesharecdn.com/deafmutism-250410181703-7e332506/85/DEAF-MUTISM-power-point-presentation-ppt-37-320.jpg)
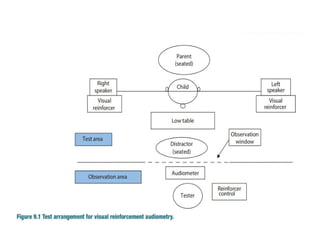
![FREE FIELD AUDIOMETRY
• Child in mothers lap is placed in between 2 loud
speakers in a sound treated room.
• Sounds [warble sound] of intensity produced
separately from speakers.
• Child turns head to the direction of the speaker
which producing sound.
• Gradually lower the intensity of sound sucessive
presentations
• Threshold of hearing can be roughly assessed.
Disadv- child loses interest becomes inattentive after 2-
3 times](https://image.slidesharecdn.com/deafmutism-250410181703-7e332506/85/DEAF-MUTISM-power-point-presentation-ppt-38-320.jpg)








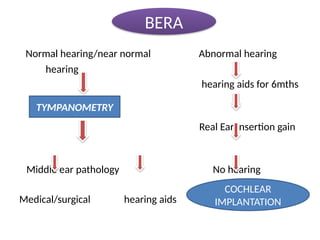
![BERA FOR THRESHOLD ESTIMATION
• Consists of presenting sound stimuli at gradually
plug /decreasing intensties and ascertaining the
minimum intensity of sound at which wave 5 of BERA
first appears.
k/a BERA threshold of the subject
if normal hearing SPL
avg PTA with in 10db 25 -30db lower
[ behavioral threshold]](https://image.slidesharecdn.com/deafmutism-250410181703-7e332506/85/DEAF-MUTISM-power-point-presentation-ppt-49-320.jpg)


















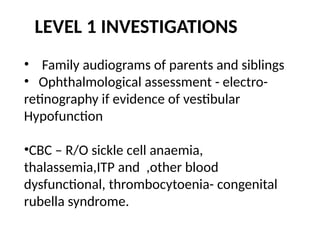
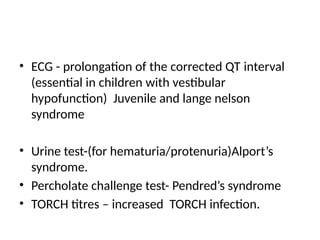
![Imaging
• MRI of Internal Auditory Meati and Brain [in
SNHL] orCT Scan of Petrous Temporal bone [in
permanent CHL] Both MRI and CT in bacterial
meningitis.
• HRCT Dilated ventricular adequate(MC)
Mondini dysplasia
Narrow IAC
Abnormality of scc
MRI –indicated only in patient for cochlear
implantation.](https://image.slidesharecdn.com/deafmutism-250410181703-7e332506/85/DEAF-MUTISM-power-point-presentation-ppt-74-320.jpg)
![LEVEL 2 INVESTIGATIONS
•Serology [Syphilis, HIV, Rubella, Toxoplasma].
•Haematology/Biochemistry – Not recommended
as routine, (TFT -if Family h/o thyroid disease,
Goitre or Mondini deformity)
• Investigation into autoimmune disease..
•Metabolic screen on blood and urine (if
epilepsy,Neuroregression).](https://image.slidesharecdn.com/deafmutism-250410181703-7e332506/85/DEAF-MUTISM-power-point-presentation-ppt-75-320.jpg)











![COMMUNICATION OPTIONS &
INTERVENTION
• Verbal strategies
- Auditory (Amplification or Cochlear
implantation or Auditory brain stem
implantation
- Non auditory [ Lip reading]
• Non verbal strategies [ sign language]](https://image.slidesharecdn.com/deafmutism-250410181703-7e332506/85/DEAF-MUTISM-power-point-presentation-ppt-87-320.jpg)