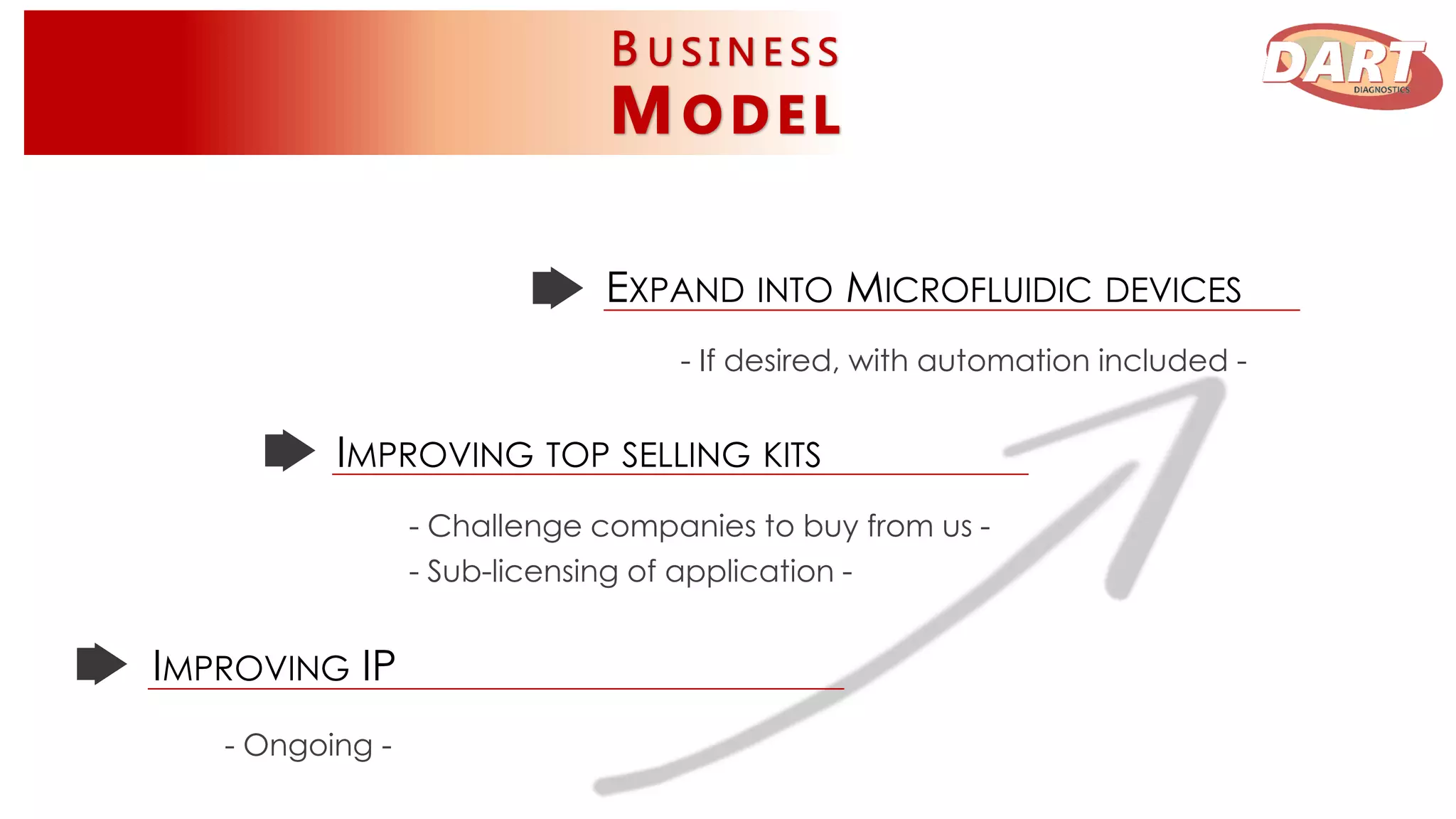
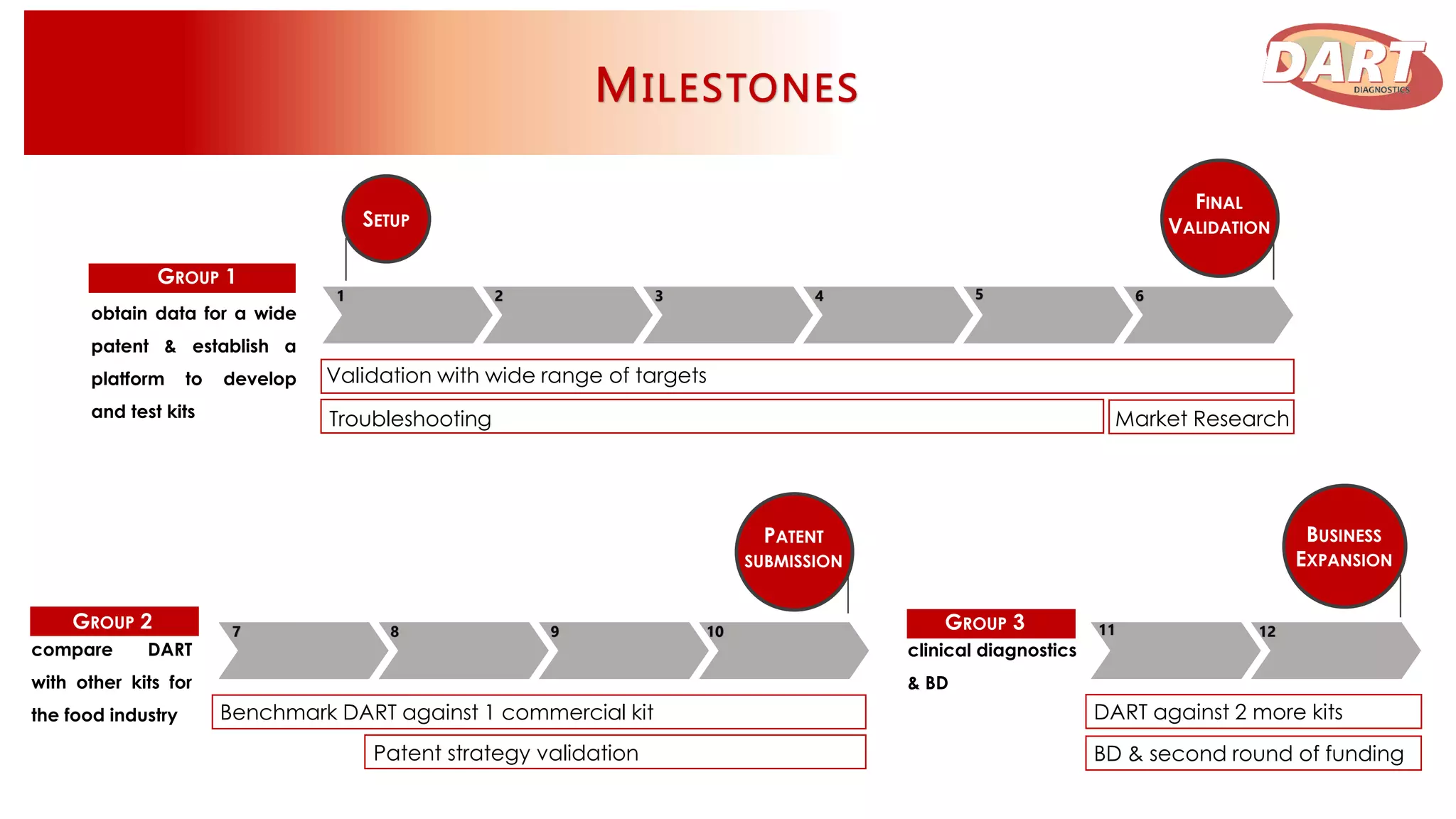
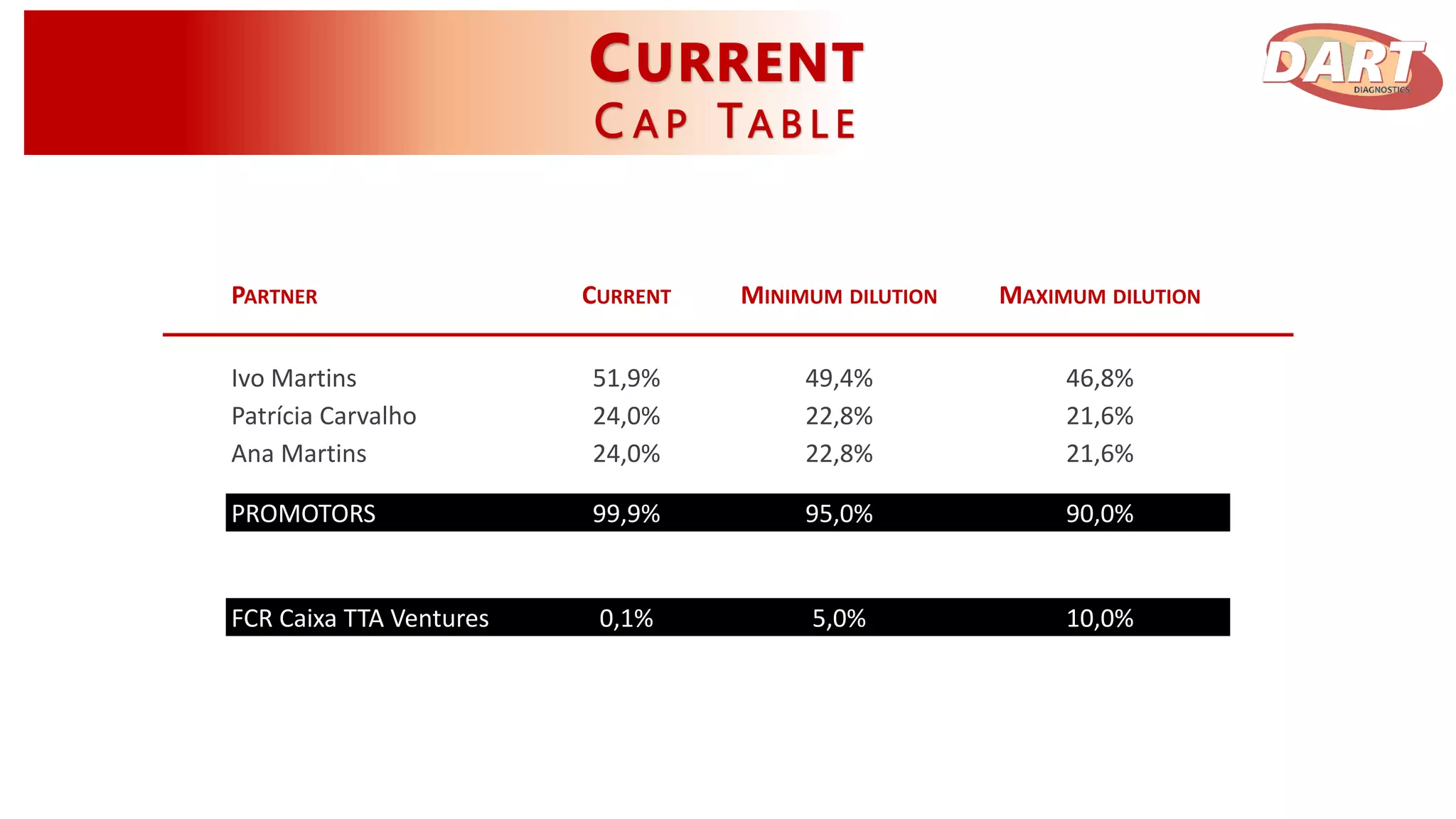
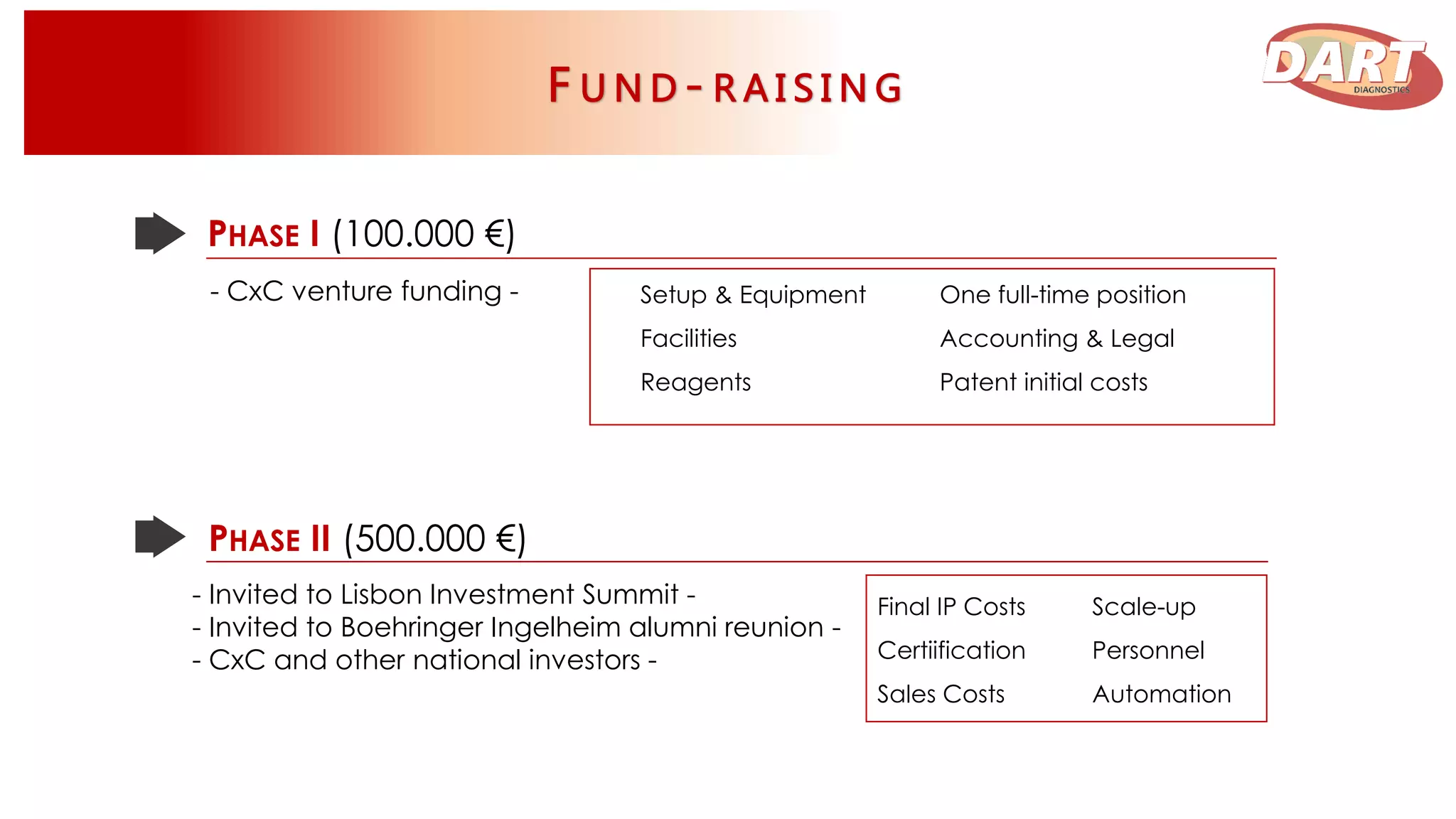
This document discusses Dart Diagnostics, a startup developing a modular plug-and-play element for signal multiplication in real-time detection, valued at $14.7 billion in 2015. The founders have extensive experience and awards in areas like biophysics, biochemistry, and nanomedicine. Dart has obtained seed funding and is validating its technology by comparing it to commercial kits for food safety and clinical diagnostics. Upon further validation and patenting, the company aims to expand into microfluidic devices, seek additional funding, and pursue partnerships, licensing agreements, or acquisition by a pharmaceutical/diagnostics firm.