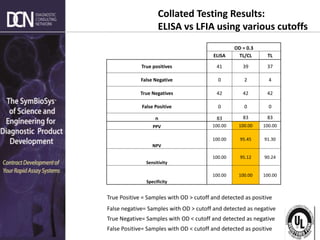
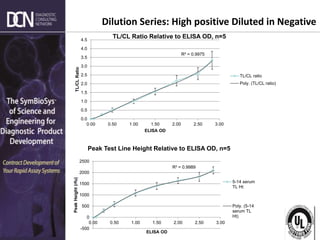
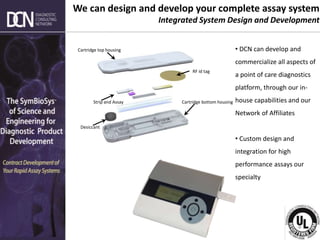
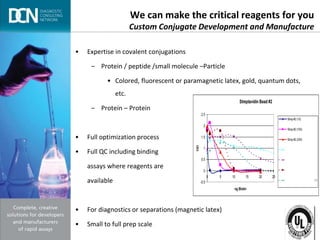
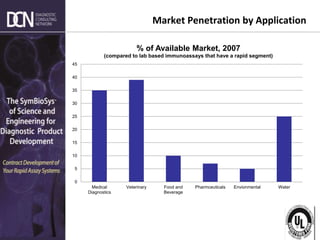
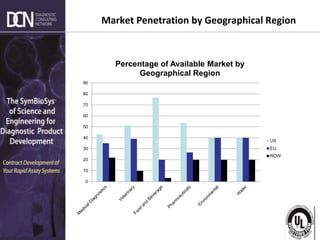
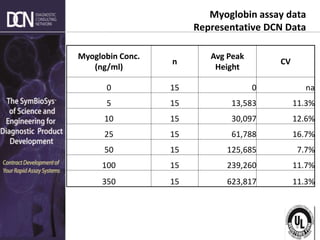
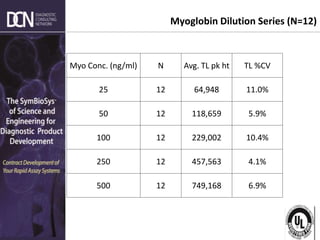
This document provides an overview of rapid diagnostic testing technologies and market opportunities. It discusses:
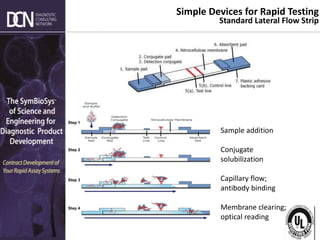
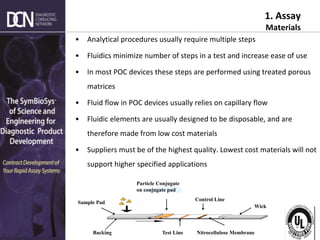
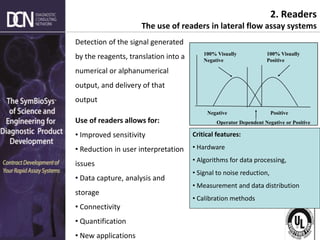
1) The state of rapid assay technologies, including standard lateral flow and more advanced formats for quantification and sensitivity.
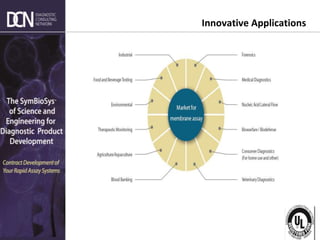
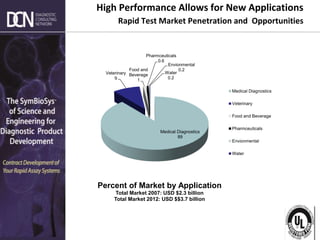
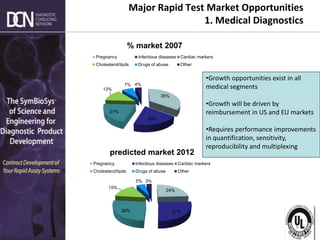
2) The major market segments and growth opportunities for rapid testing in medical diagnostics, veterinary, food/beverage, and environmental applications.
3) How market demands are driving technological innovation to improve assay performance characteristics like sensitivity and multiplexing.






















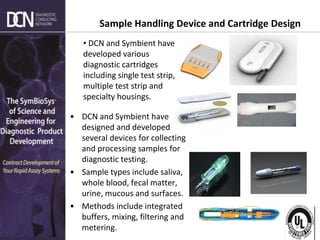
![Complete, creative solutions for developers and manufacturers of rapid assays
• Sensitive
• Affordable
• Simple to operate
• Small, Mobile and Robust
• Documented Results
• Objective Results
• Quantitative Results
ESE Quant
DCN’s Reader Partners: Handheld and Benchtop Readers
DCN distributes both ESE and LRE readers
DCN acts as the assay integration partner for both suppliers for
customers worldwide
LRE POC Reader
0
200
400
600
800
1000
1200
1400
0 200 400 600 800 1000 1200 1400
Data points
Intensity
[mV]
Scan1
Scan2
Scan3
Scan4
Scan5
Scan6
Scan7
Scan8
Scan9
Scan10
Scan11
Scan12
Scan13
Scan14
Scan15
Scan16
Scan17
Scan18
Scan19
Scan20
Control
Test](https://image.slidesharecdn.com/dcninovatecpresentationfinal-101110124949-phpapp01/85/Dcn-inovatec-presentation-final-31-320.jpg)





![Complete, creative solutions for developers and manufacturers of rapid assays
Alpha Amylase Detection in Wheat
0
50
100
150
200
250
300
350
400
srw170 srw138 srw139 cbh2.7 cbh2.8
Falling
Number
Wheat Sample
Comparisonof Values from Device andTrue Falling
NumberValues
True FN
DiagnostIQ AvgFN
DiagnostIQ Avg
[Amylase]
Hagberg Falling Numbers are determined by dropping a ball through a tube of
ground wheat. The time it takes in seconds for it to reach the bottom correlates
inversely to the amount of alpha-amylase in the wheat. High falling numbers
indicate low concentrations of alpha-amylase, and low falling numbers indicate a
high concentration of alpha-amylase.
Falling Number
True
FN
avg
amyl
conc.
St
Dev %CV
304 1.54 6.4 1.77%
244 21.82 3.56 1.27%
223 36.02
16.8
4 6.77%
142 144.05 6.57 4.77%](https://image.slidesharecdn.com/dcninovatecpresentationfinal-101110124949-phpapp01/85/Dcn-inovatec-presentation-final-42-320.jpg)