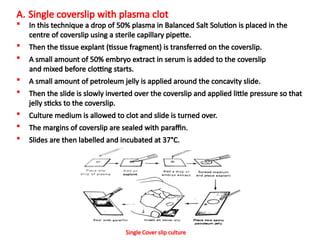
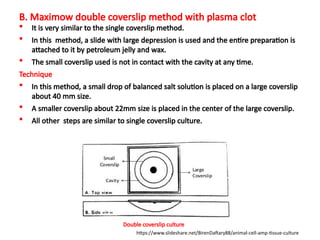
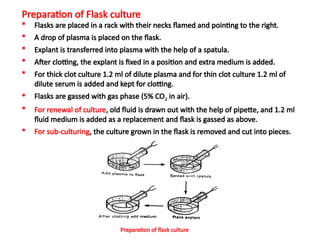
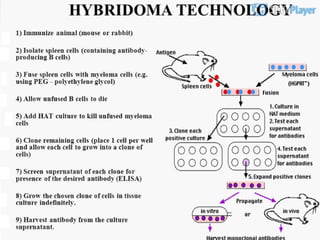
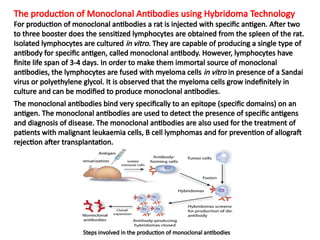
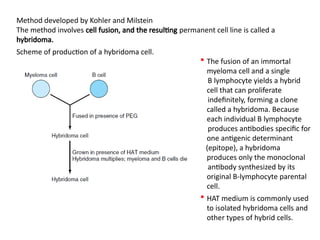
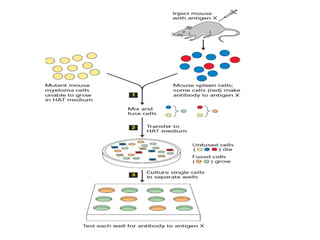
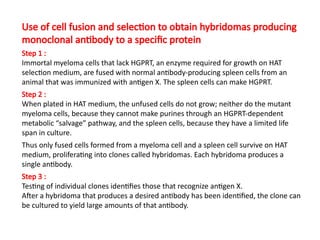
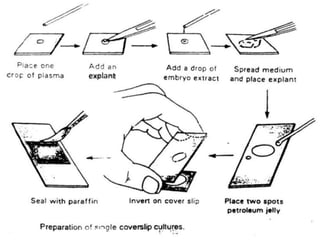
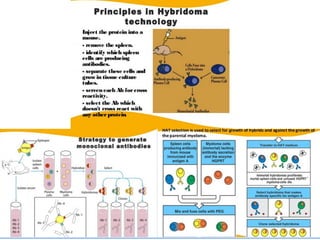
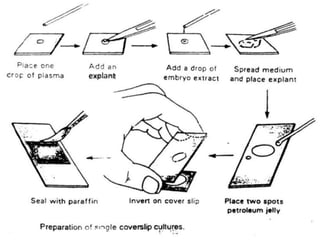
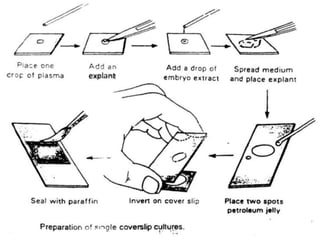
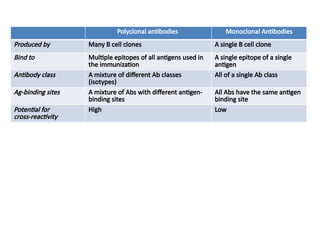
The document discusses various culture techniques for cells and organs, detailing the preparation methods, types of cultures (such as slide, flask, and tube), and the differences between primary and established cell lines. It highlights the procedures for creating viable cell cultures, methods of disaggregation, and the utilization of hybridoma technology for monoclonal antibody production. Additionally, it addresses the advantages and disadvantages of different culture techniques and the characteristics of primary versus established cell lines.