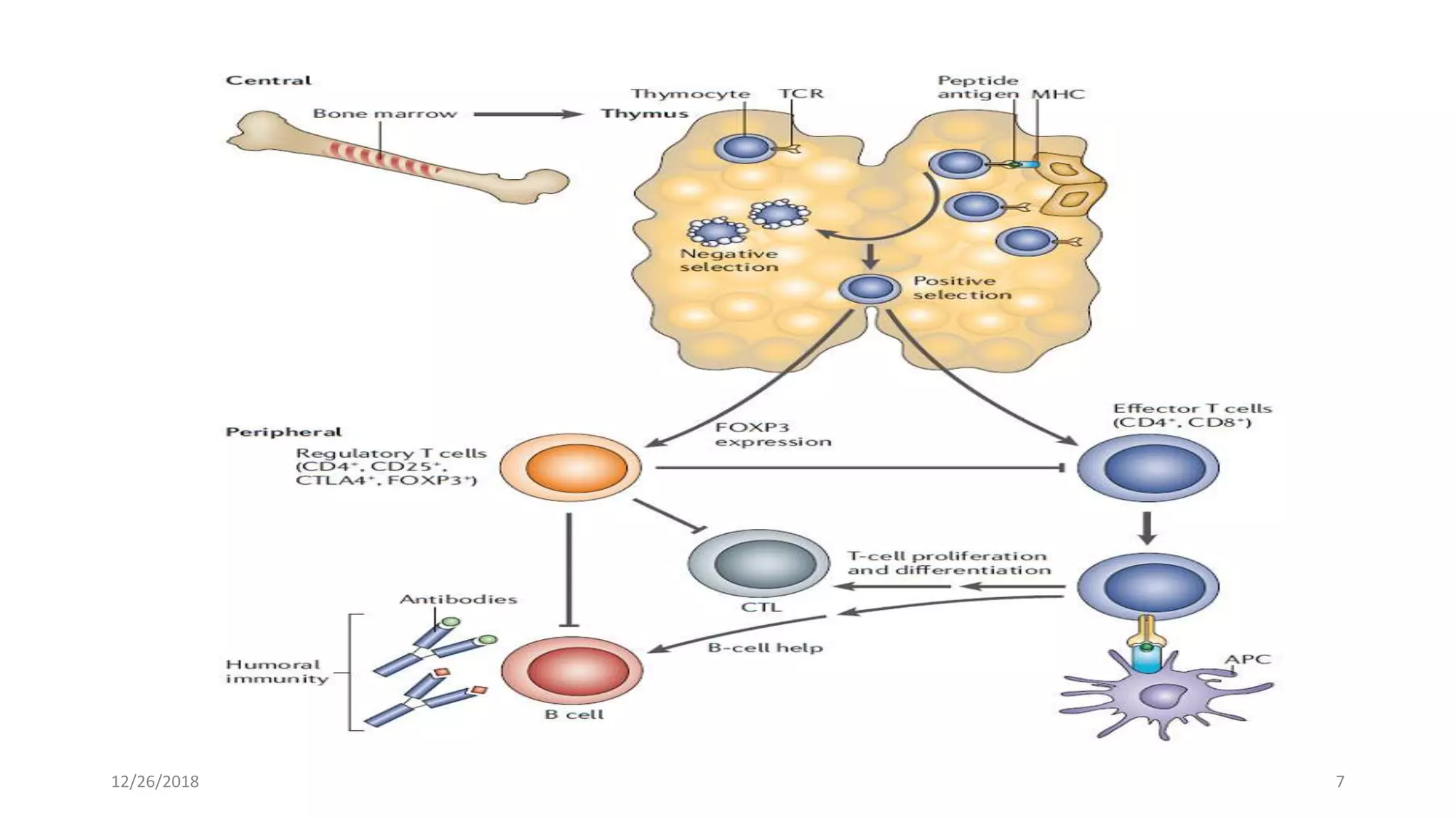
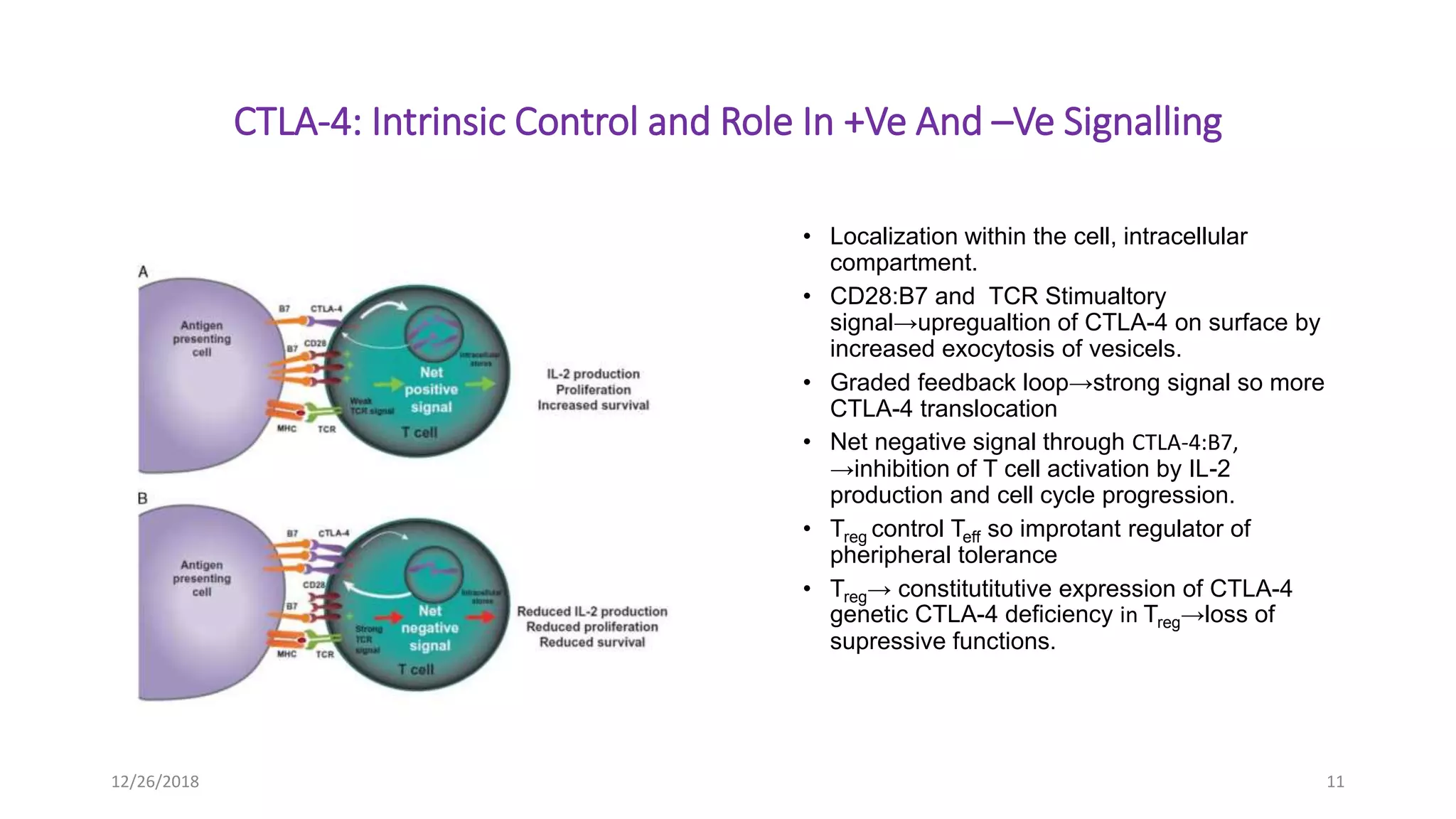
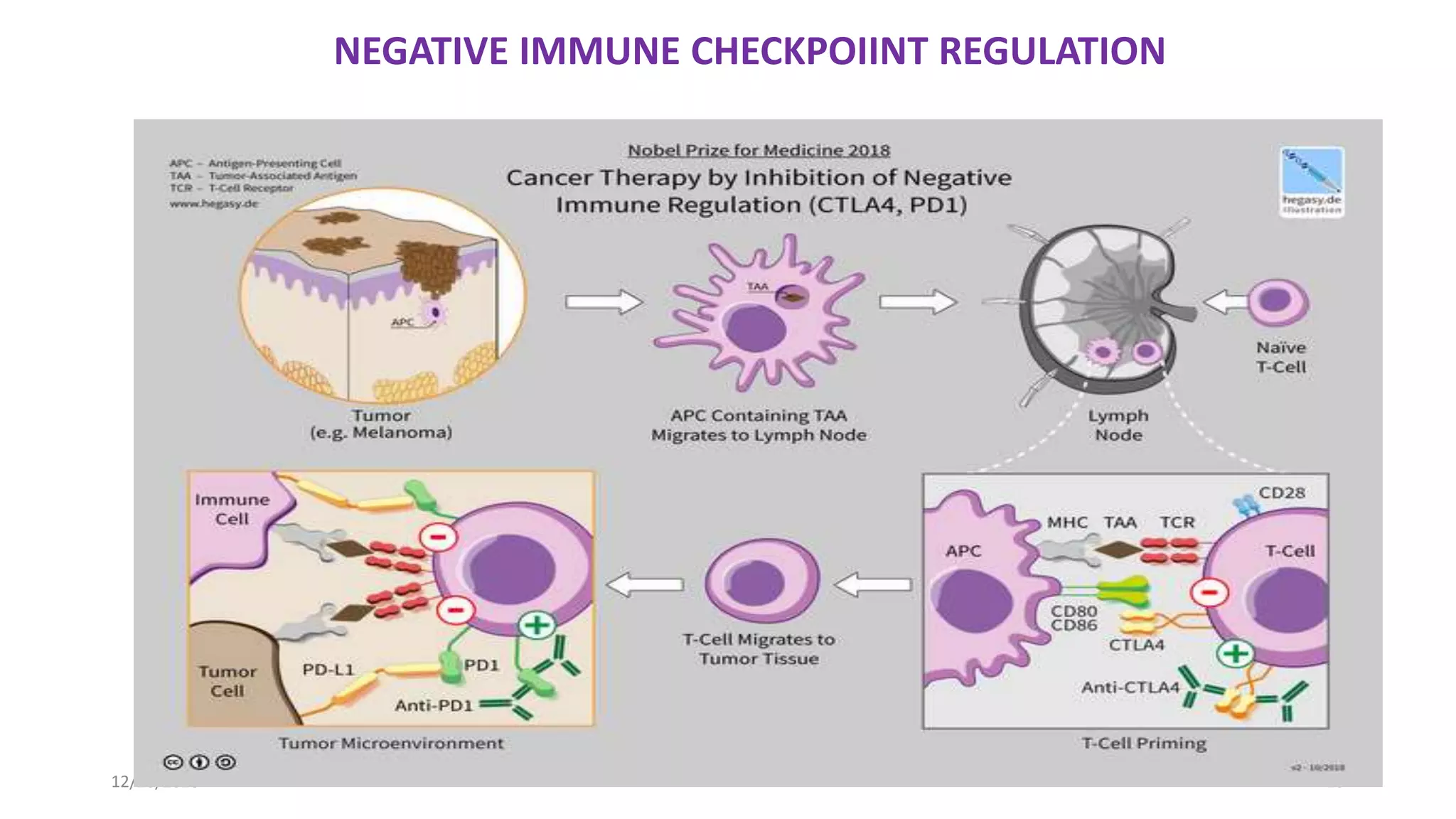
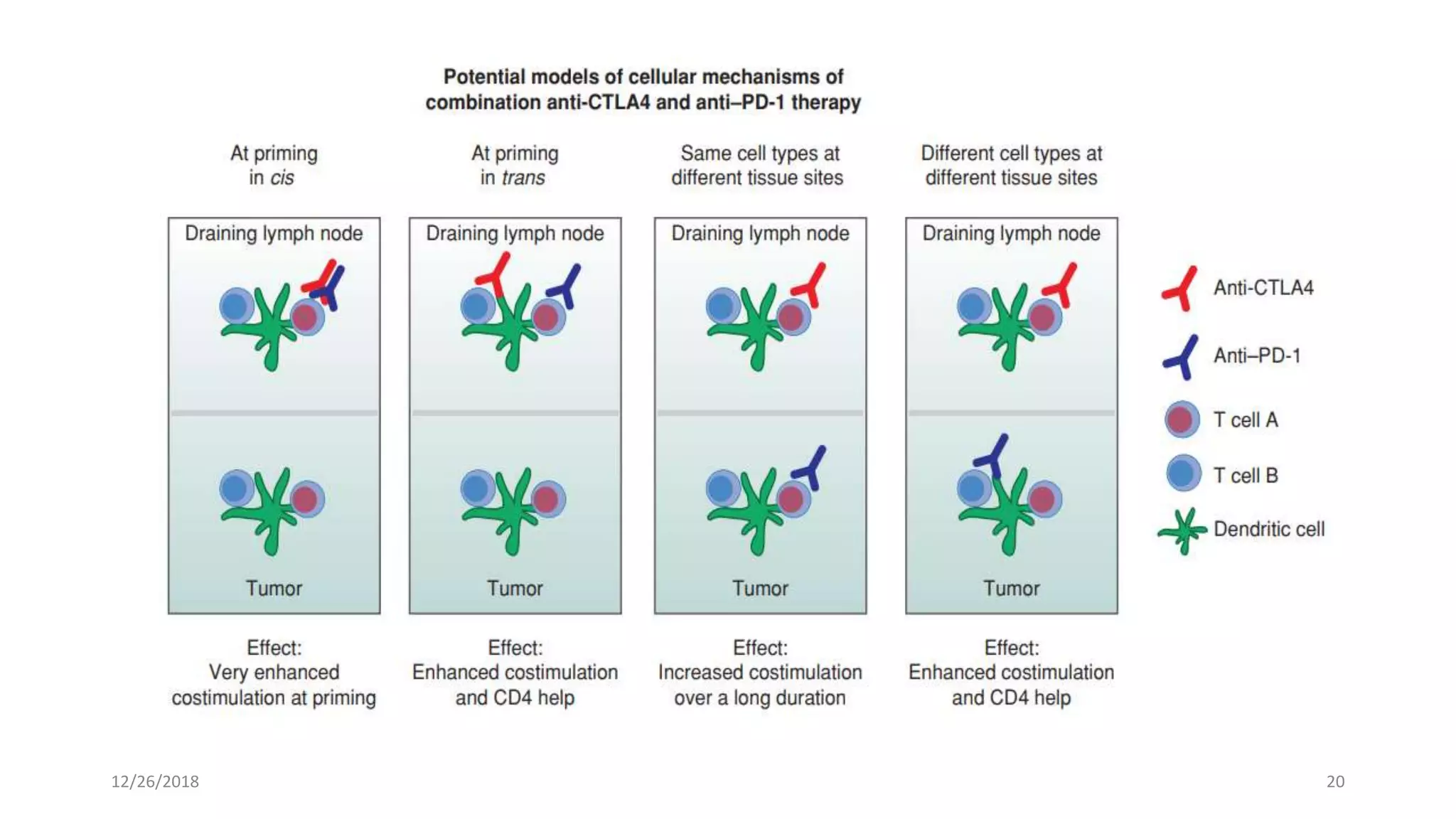
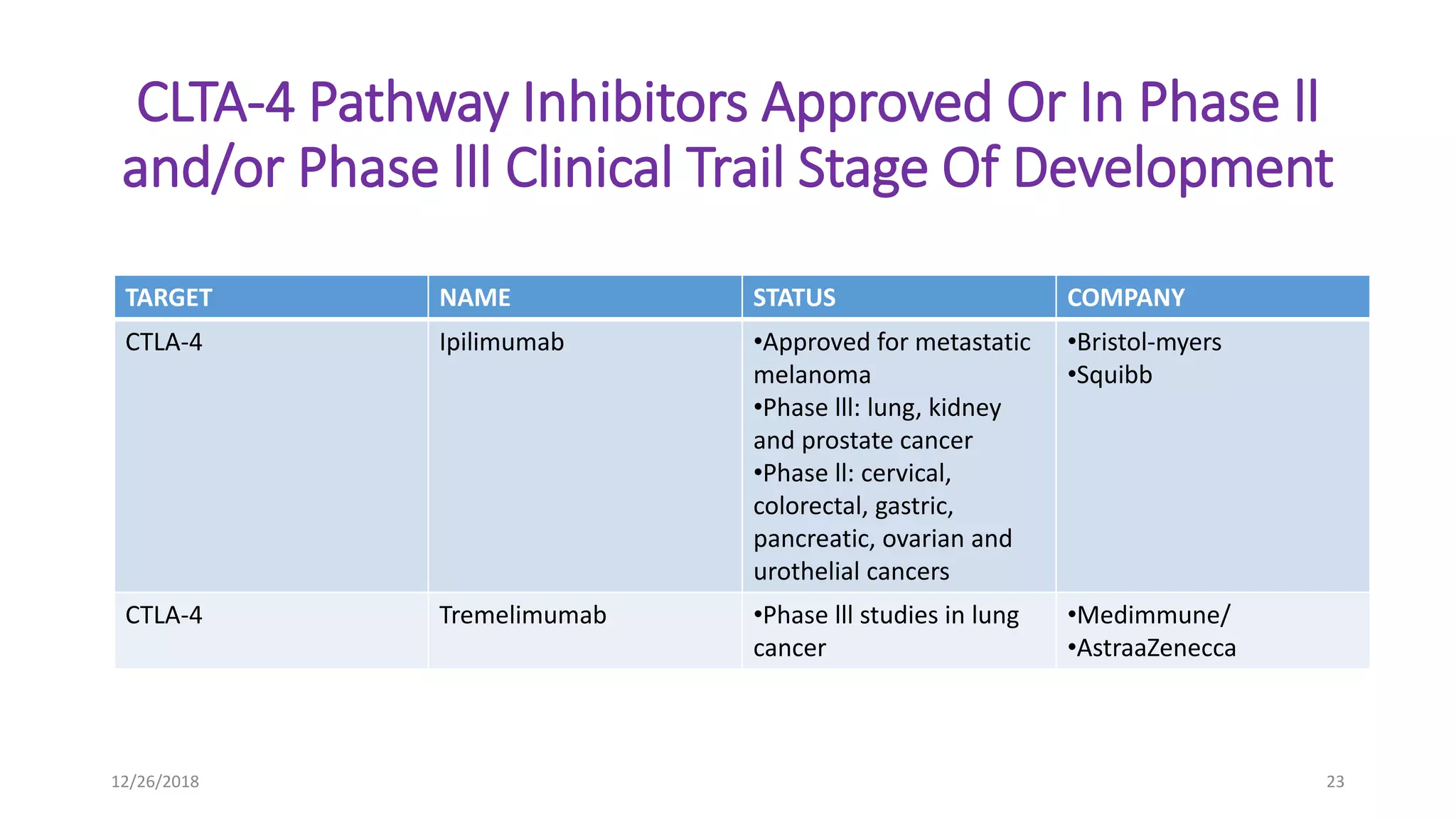
The document discusses the history and function of cytotoxic T-lymphocyte antigen 4 (CTLA-4) as an immune checkpoint regulator that inhibits T-cell activation and promotes peripheral tolerance. It covers the mechanisms of immune checkpoint blockade therapy, particularly the use of CTLA-4 inhibitors like ipilimumab and tremelimumab in cancer treatment, enhancing anti-tumor immune responses. Finally, it highlights clinical implications, biomarkers, and the associated immunologic toxicities of these therapies.