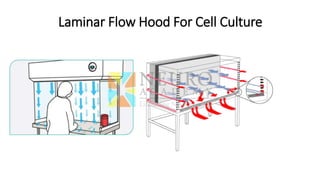
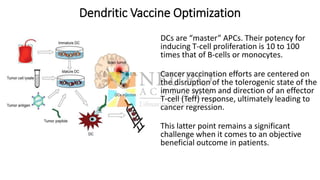
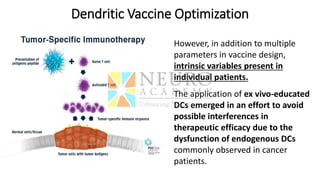
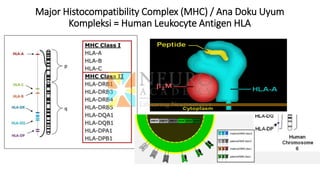
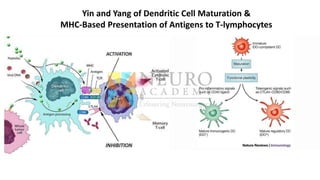
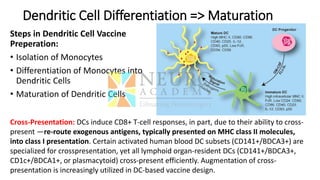
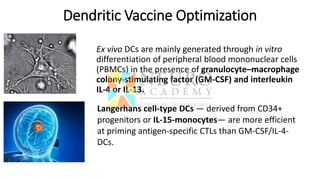
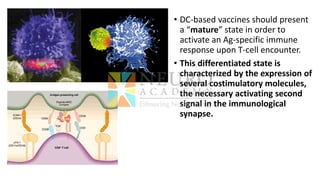
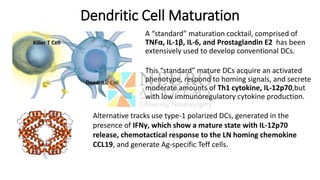
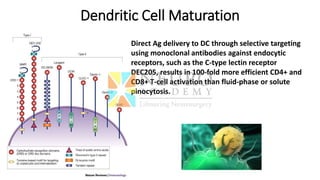
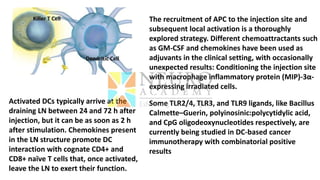
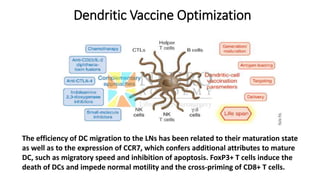
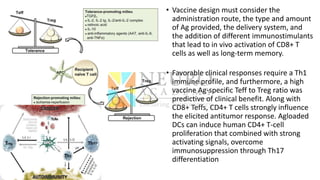
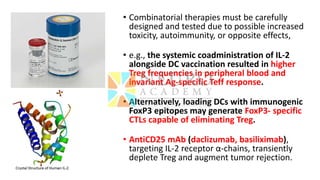
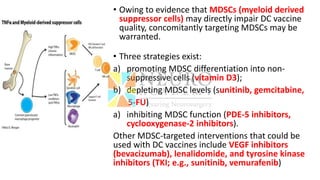
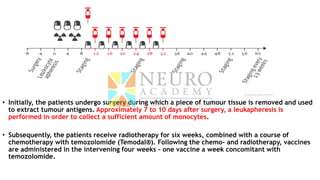
This document discusses optimization of dendritic cell vaccines for cancer immunotherapy. It covers topics such as isolating monocytes and differentiating them into dendritic cells, maturation of dendritic cells, factors that influence cross-presentation of antigens to T-cells, and adjuvants that can enhance the immune response. Key challenges mentioned are inducing an effector T-cell response against cancer cells while overcoming immunosuppression in cancer patients. The document also discusses combination therapies and strategies for targeting myeloid-derived suppressor cells and regulatory T-cells.