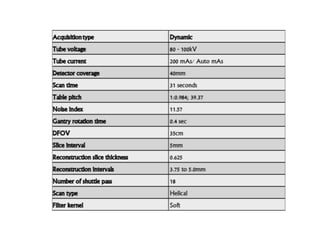
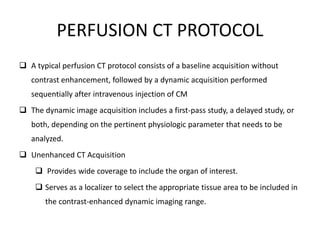
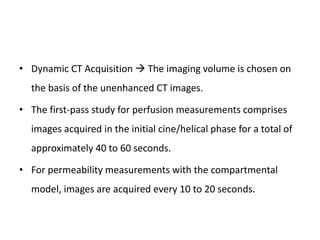
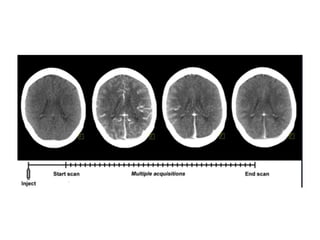
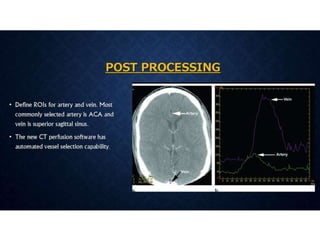
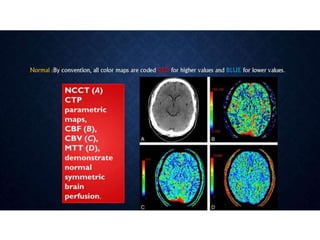
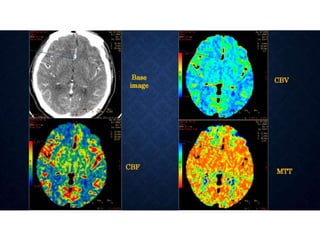
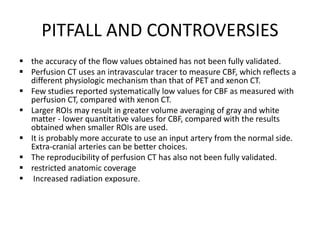
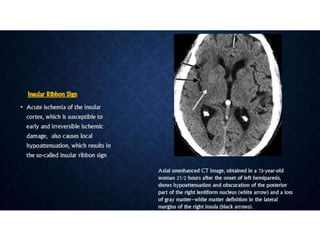
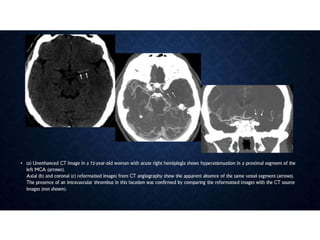
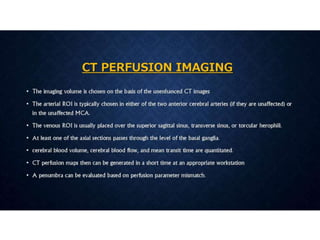
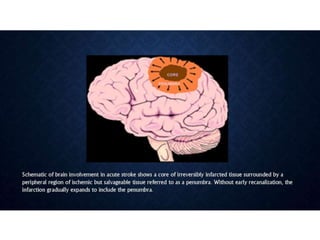
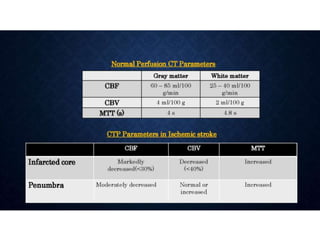
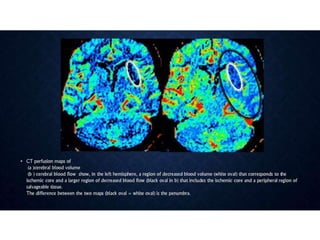
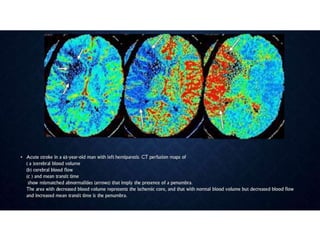
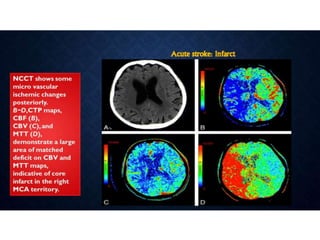
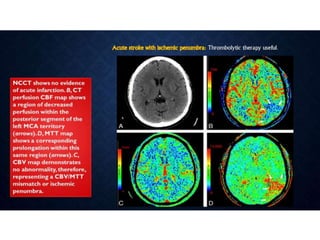
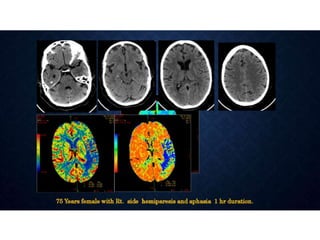
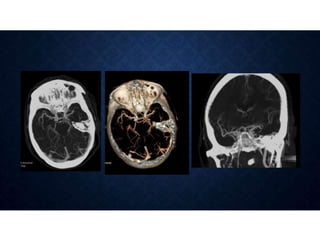
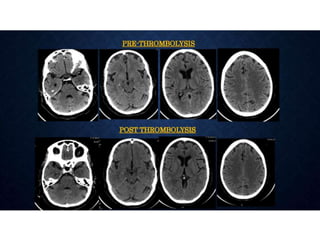
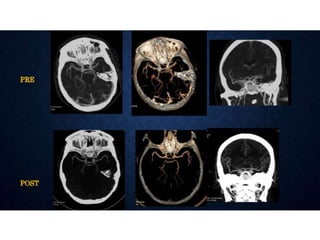
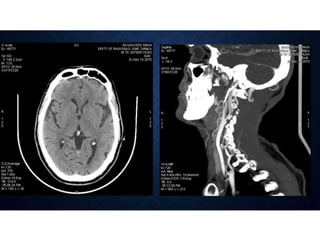
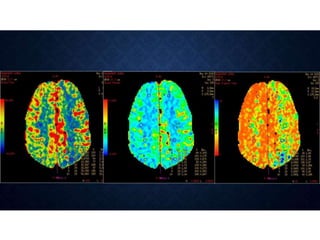
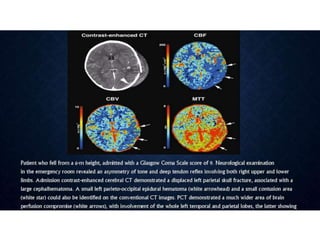
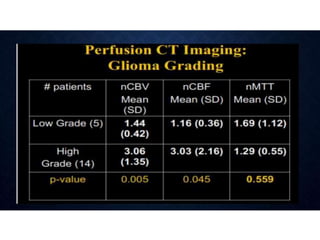
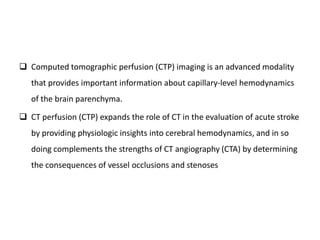
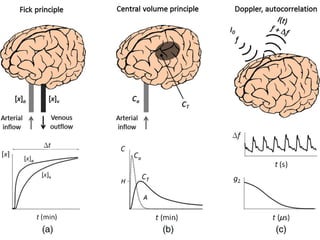
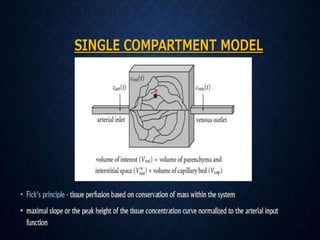
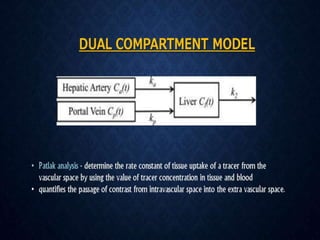
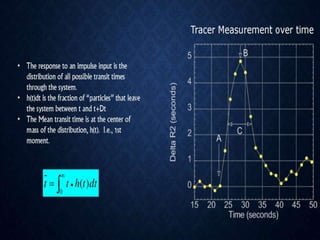
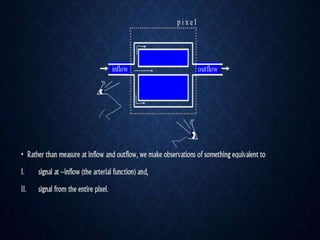
CT perfusion (CTP) provides important hemodynamic information about cerebral blood flow, blood volume, and mean transit time that complements CT angiography in the evaluation of acute stroke. CTP can help characterize acute ischemic stroke by identifying critically ischemic tissue in the stroke "core" and potentially salvageable tissue in the "penumbra", guiding treatment decisions about thrombolysis within time windows. A typical CTP exam involves an initial non-contrast CT followed by dynamic contrast-enhanced imaging over 40-60 seconds to generate perfusion maps and quantify key parameters.















![TECHNICAL IMPLEMENTATIONS
The baseline CT study should have 3 components:
- unenhanced CT,
- vertex to-arch CT angiography (CTA),
- dynamic first-pass CTP
• cardiac MDCT detection of possible left atrial appendage thrombus is
[optional]](https://image.slidesharecdn.com/ctperfusion-200224050817/85/CT-perfusion-28-320.jpg)