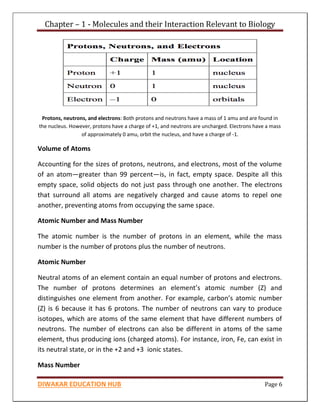
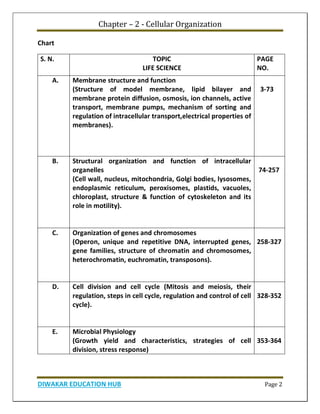
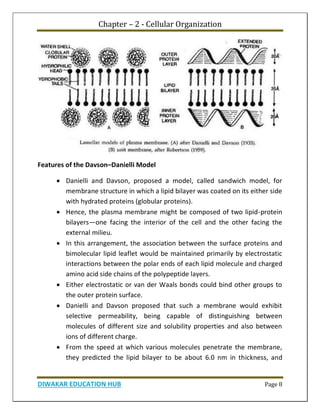
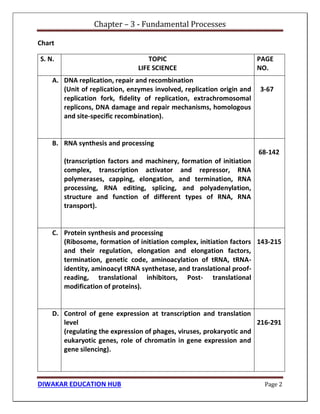
Chapter 1 discusses the structure of atoms, molecules, and their interactions, emphasizing the significance of atomic structure in biological processes. It covers the composition and functions of biomolecules, the principles of biophysical chemistry, and the basics of catalysis and enzyme kinetics. The chapter also outlines the historical advancements in atomic theory and the contributions of notable scientists to our understanding of atomic structure.
































![Chapter – 3 - Fundamental Processes
DIWAKAR EDUCATION HUB Page 5
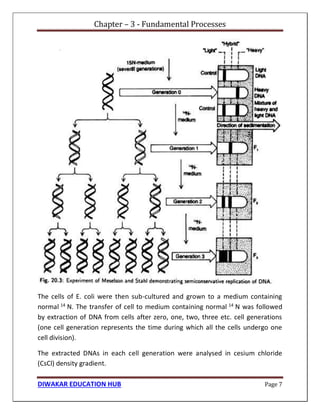
Each strand, acting as template, synthesizes their complementary new strand on
itself taking raw materials from the nuclear sap. Thus two daughter DNA helices
are formed. Each daughter DNA helix has one old or parental and one new strand.
It indicates that in each daughter DNA helix, one parental strand is retained and
conserved while its complementary strand is new. Hence, according to this mode
of DNA replication, the parental DNA is partially conserved in each new daughter
DNA molecule. So this mode of DNA replication is called semiconservative
replication [Fig. 20.2(a)].](https://image.slidesharecdn.com/freesamplecsirnetlifescience1-221017122619-cc591fda/85/CSIR-NET-Life-Science-book-pdf-Sample-37-320.jpg)