1) Blinding in clinical trials refers to keeping trial participants, investigators, and assessors unaware of treatment assignments to prevent bias.
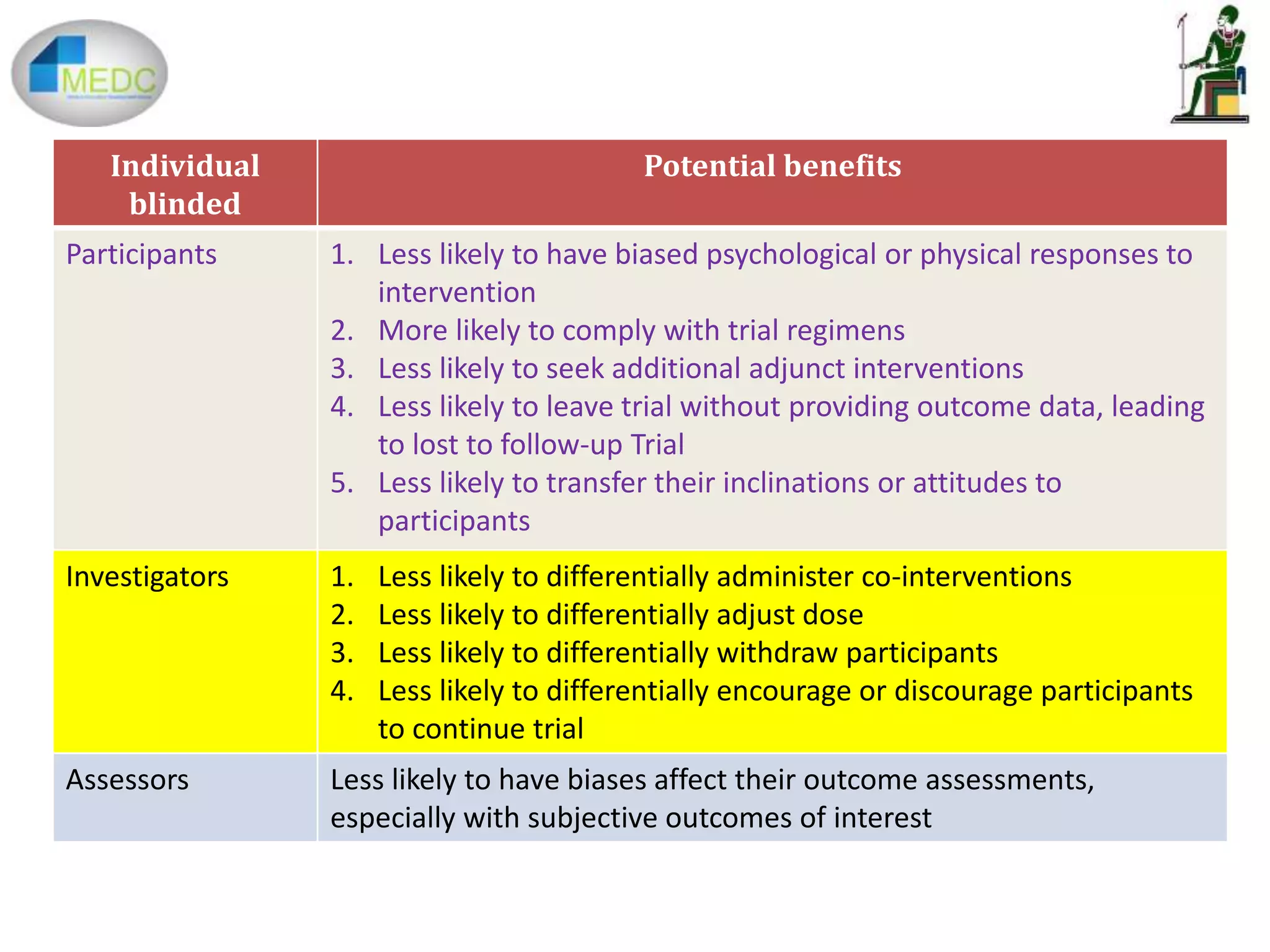
2) Potential benefits of blinding include less psychological or physical bias in participants, better compliance, and less bias in outcome assessments.
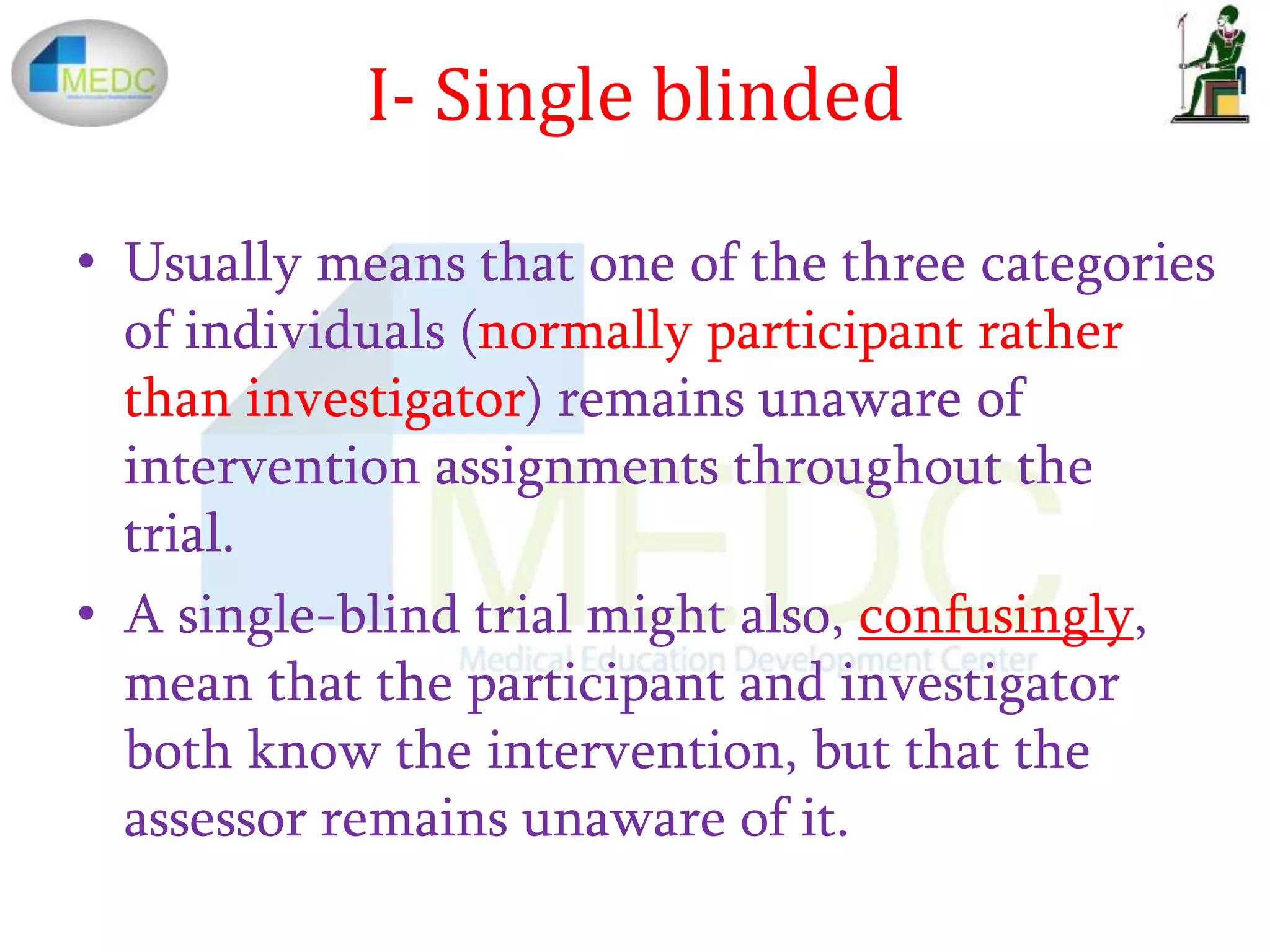
3) Types of blinding include non-blinded (where all know assignments), single-blinded (one group remains unaware), and double-blinded (participants, investigators, and assessors remain unaware). Placebos are often used to maintain blinding.