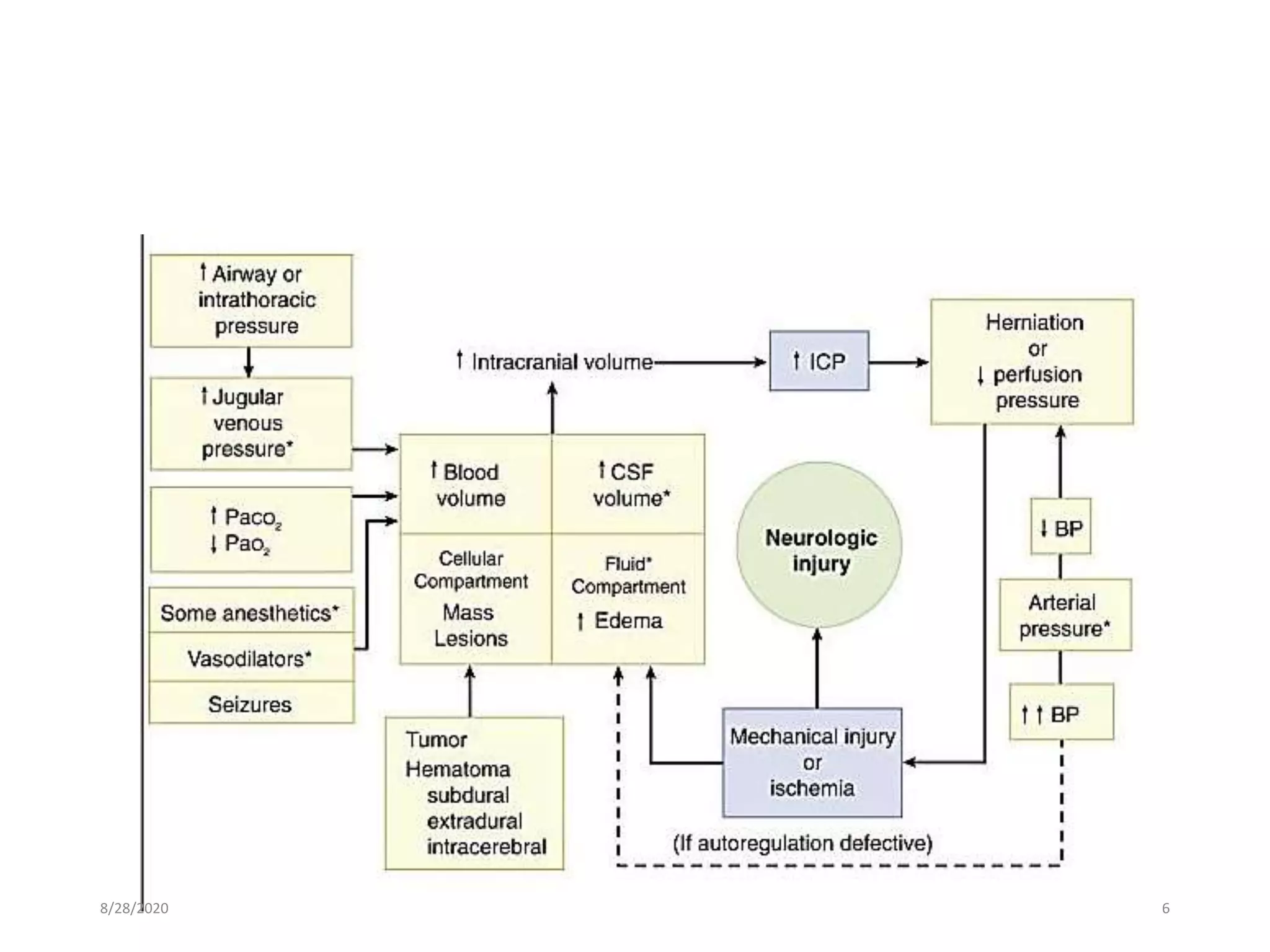
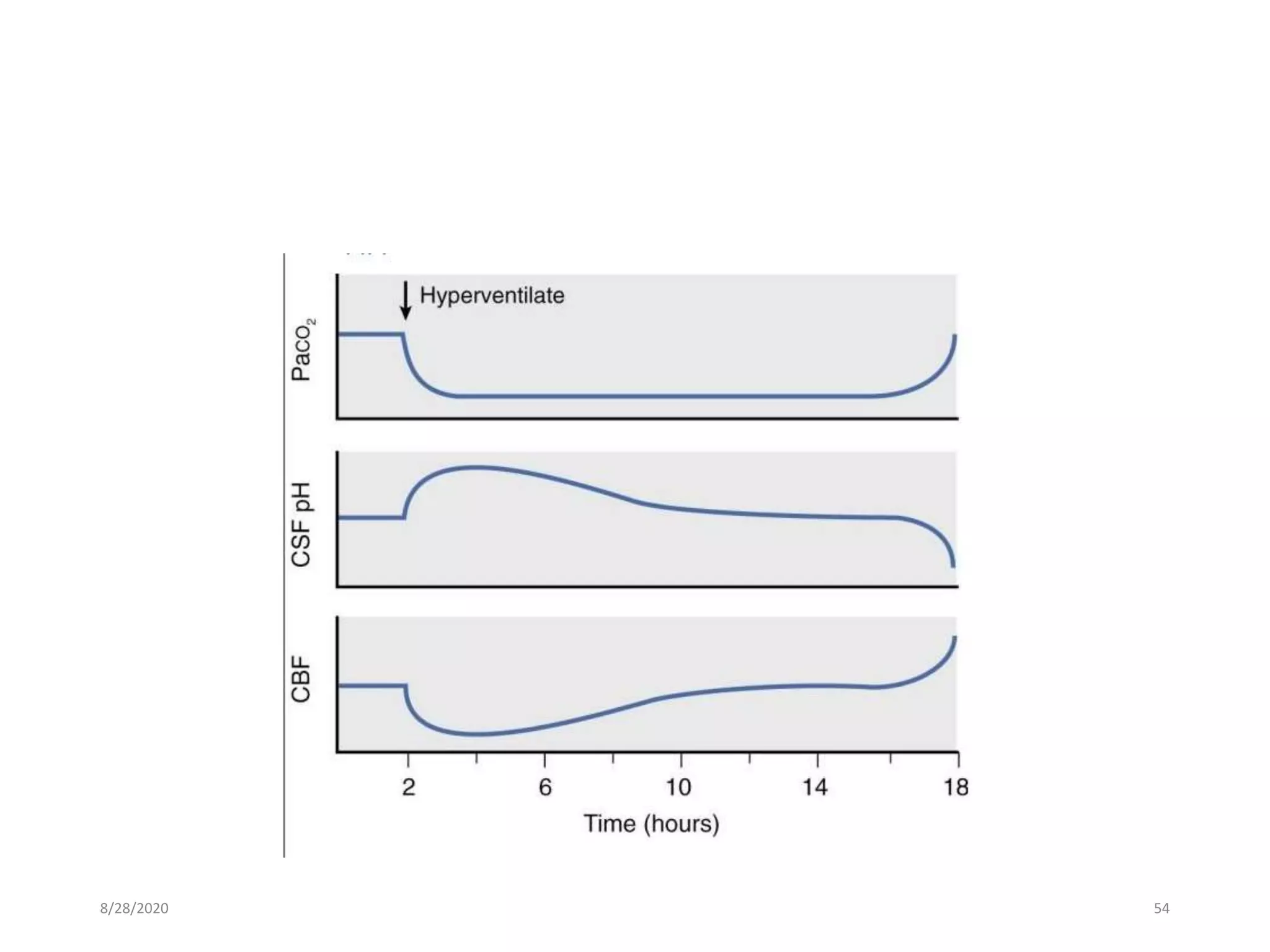
This document discusses anaesthesia considerations for craniotomy to remove a mass lesion in the brain. It covers preoperative evaluation focusing on signs of increased intracranial pressure. Strict control of blood pressure, intubation technique to avoid pressure increases, and maintenance with balanced anaesthesia to control ICP and CPP are emphasized. Monitoring of ICP, CPP and other parameters is important. Positioning must be done carefully to avoid pressure on nerves or veins.