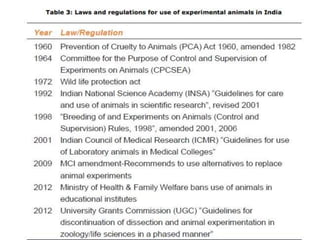
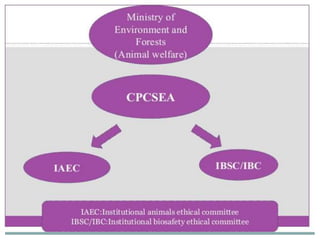
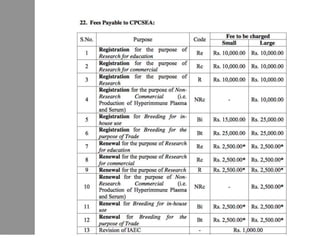
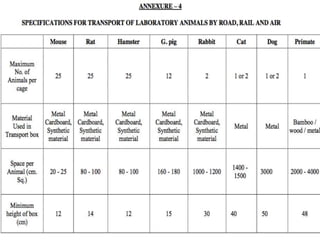
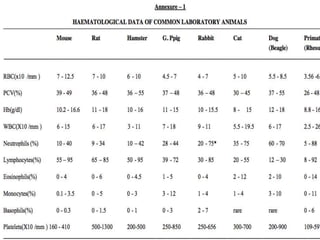
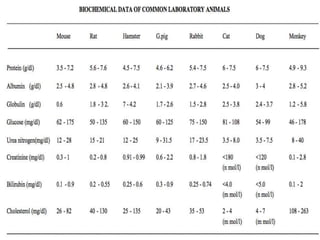
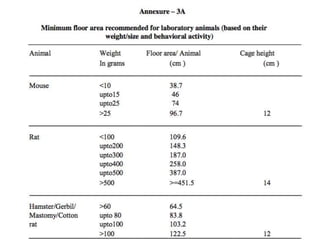
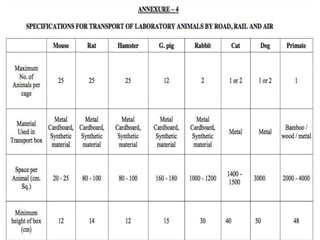
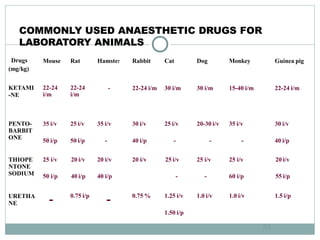
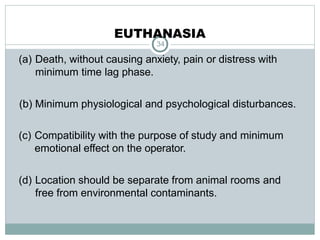
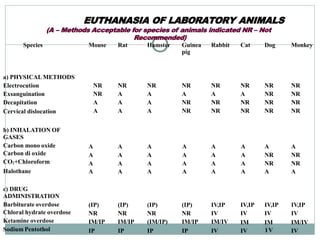
The document discusses guidelines from the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) in India. It outlines CPCSEA's aim to promote humane care of animals used in research while advancing biological knowledge. Key points include CPCSEA introducing Good Laboratory Practice standards and the 3Rs principles of replacement, reduction and refinement of animal use. Guidelines cover proper housing, care, experimental procedures, record keeping and humane euthanasia to minimize animal suffering.