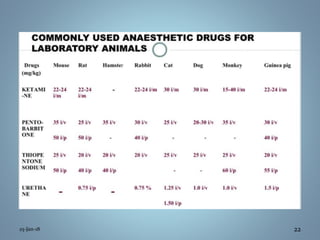
The document outlines comprehensive guidelines for the control and supervision of animal experiments, emphasizing humane treatment, health care, and proper experimental protocols. It includes directives on animal procurement, veterinary care, sanitation, and waste disposal, as well as standards for housing and environmental conditions. Additional sections cover anesthesia, euthanasia, and the roles of trained personnel in ensuring the welfare of research animals.