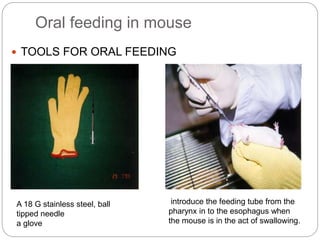
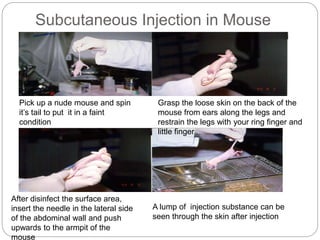
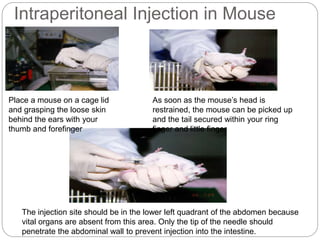
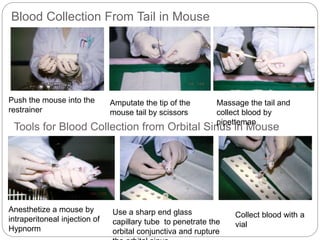
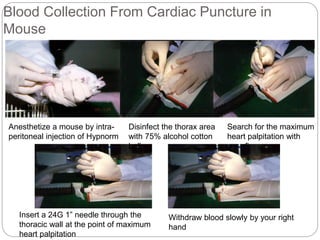
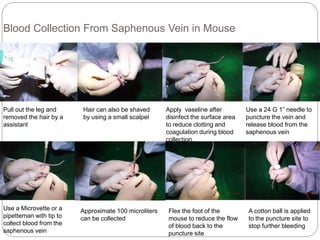
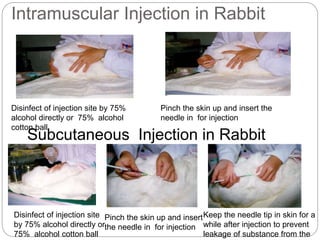
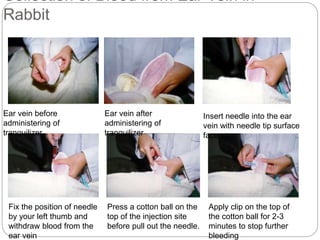
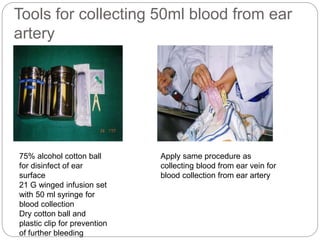
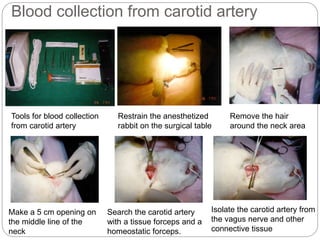
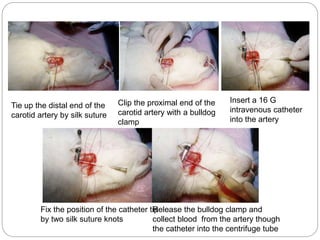
This document provides information on proper animal handling techniques. It discusses objectives of avoiding mishandling and complying with regulations. Procedures for mice include oral feeding, injections, and blood collection from various sites. Rabbit techniques include intramuscular injection, blood collection from ears or arteries. Precise restraint and use of the correct tools are emphasized to safely perform common procedures.