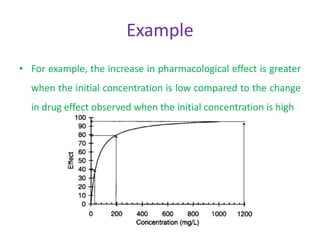
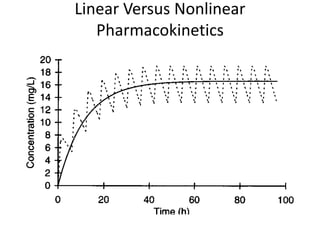
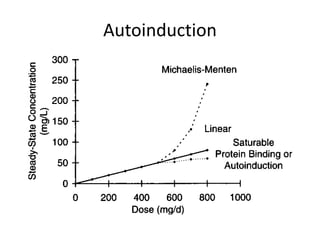
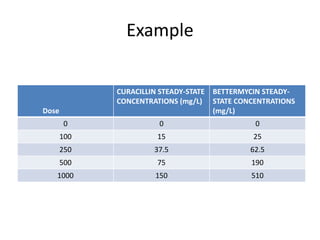
The document provides an introduction to clinical pharmacokinetics, detailing the processes of drug absorption, distribution, metabolism, and excretion. It emphasizes the importance of understanding linear and nonlinear pharmacokinetics in optimizing dosage regimens and minimizing adverse effects. Key concepts include steady state concentrations and their relationship with dosage adjustments, illustrating how some drugs may follow saturable or Michaelis-Menten pharmacokinetics.