Embed presentation
Downloaded 20 times


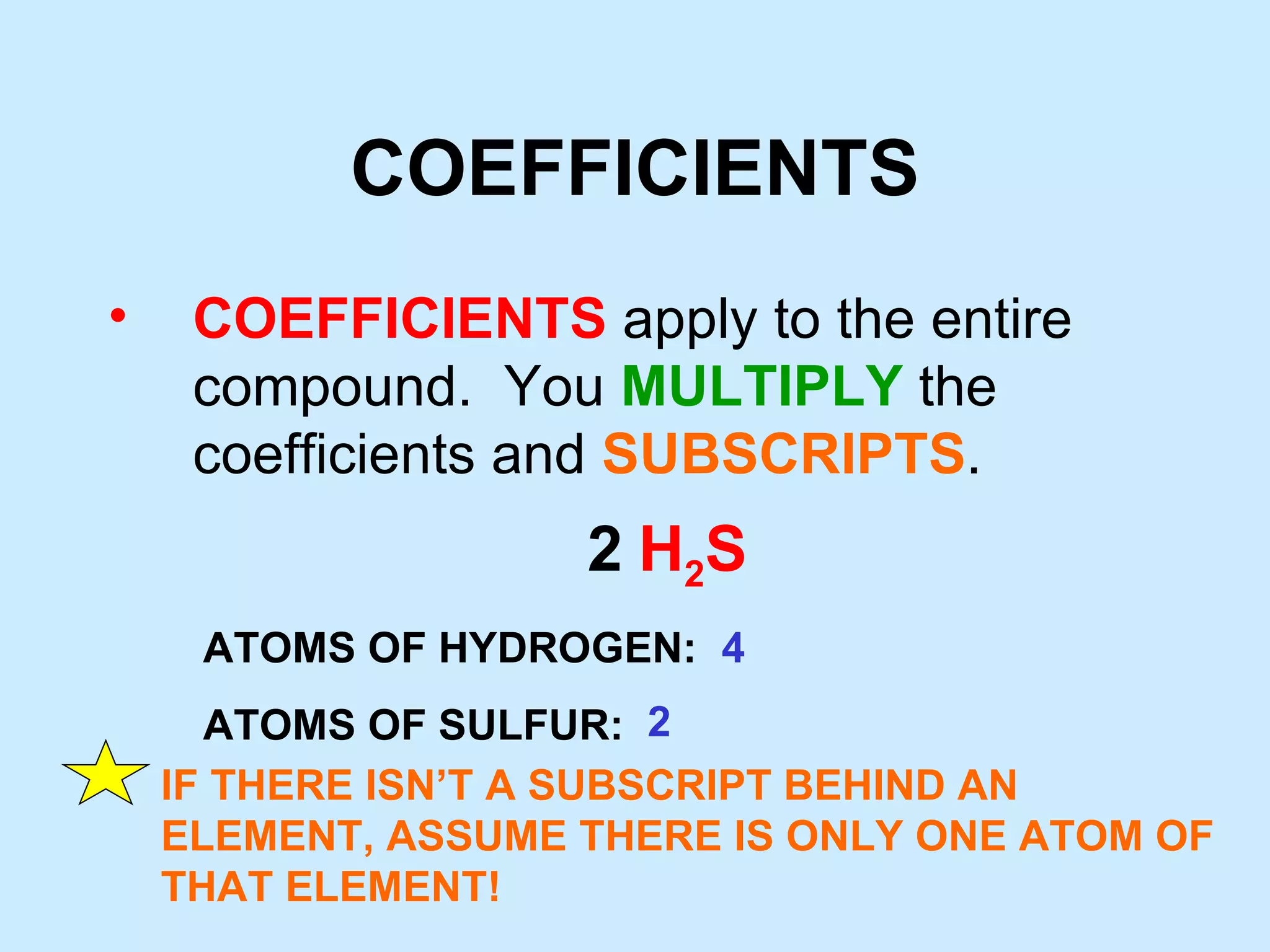
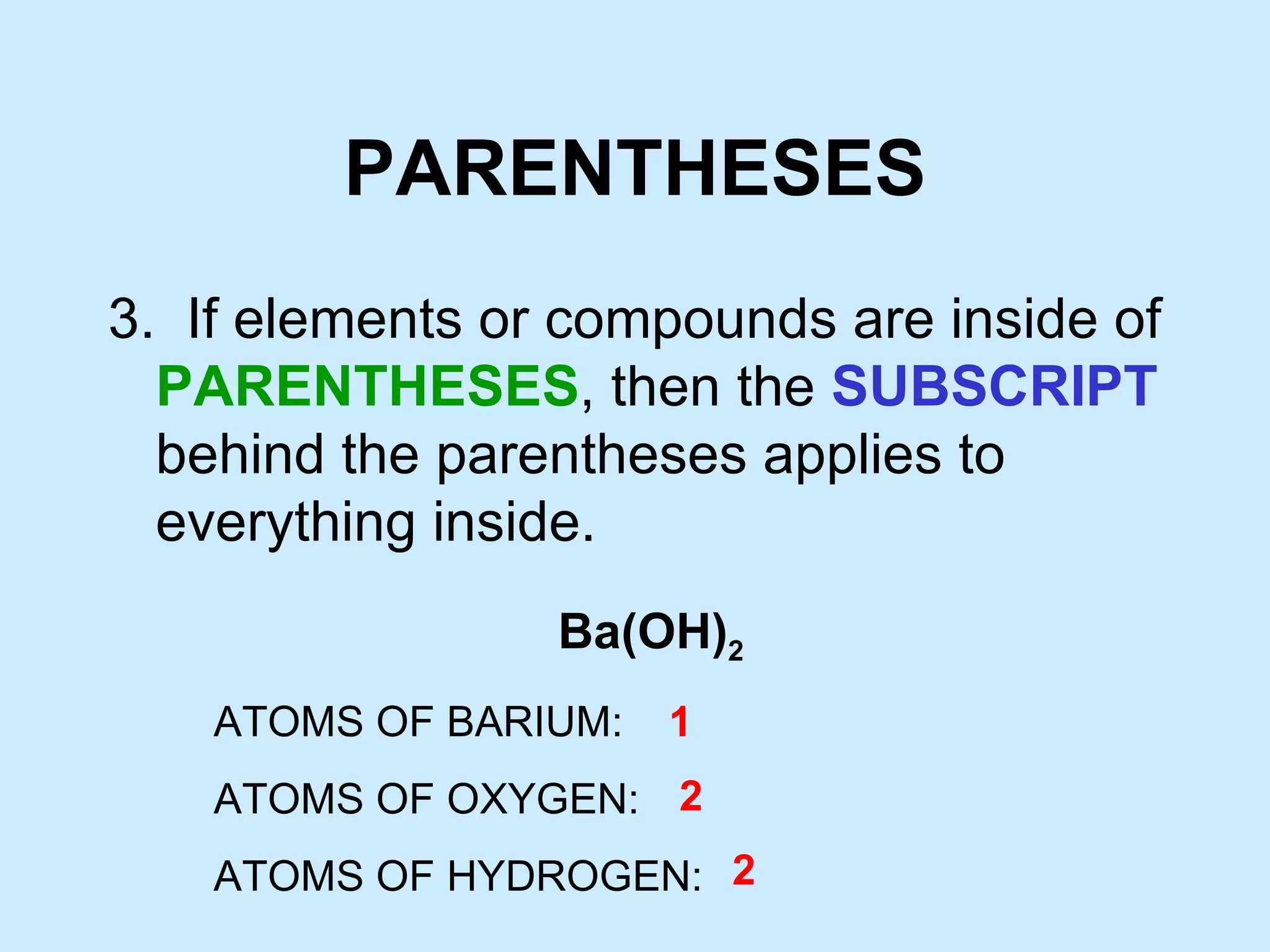

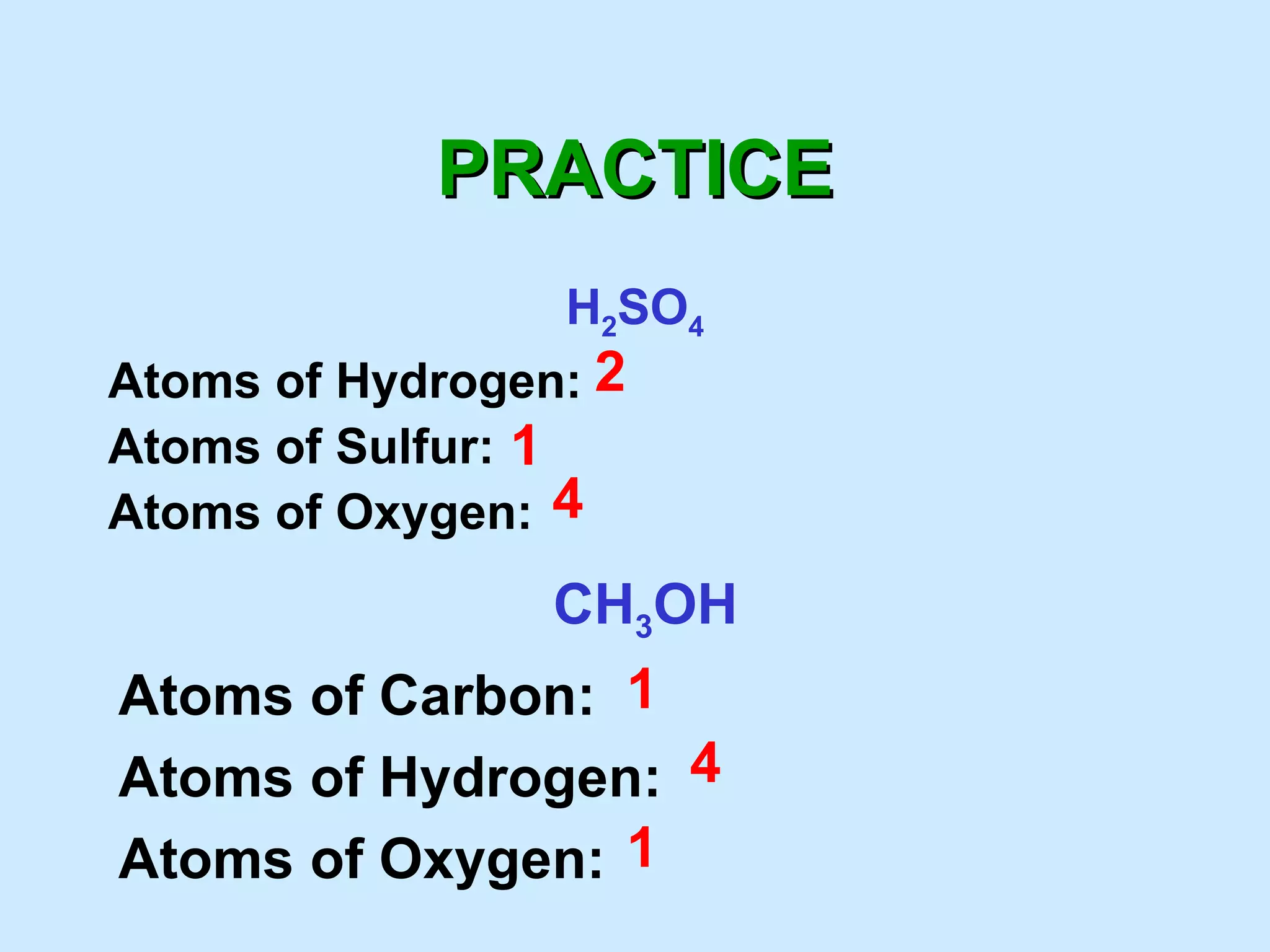

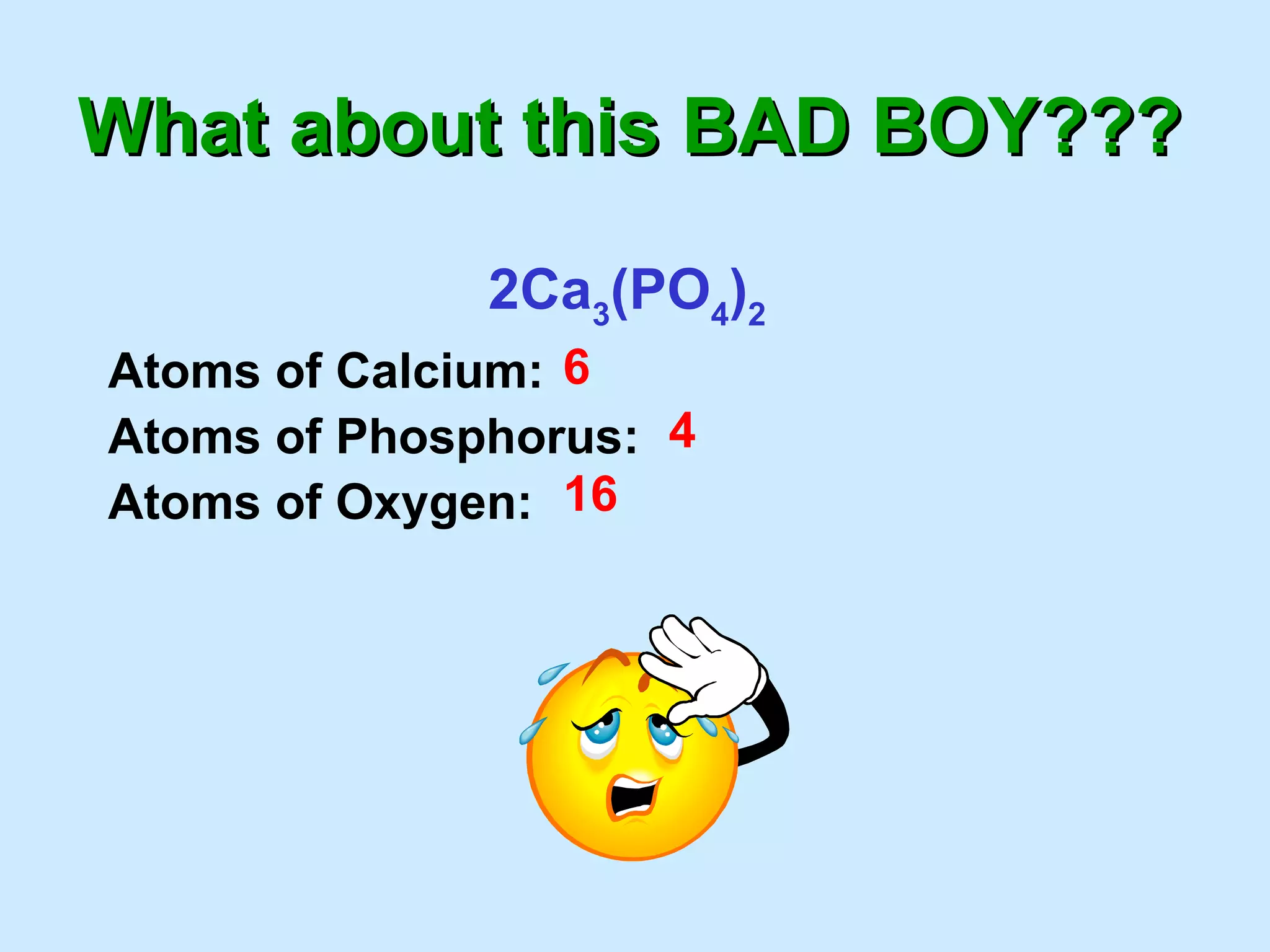

The document provides rules for counting atoms in chemical formulas including: using subscripts only for the atom behind it, multiplying coefficients and subscripts, and applying subscripts inside parentheses to all elements within. It then provides examples of using the rules to determine the number of each type of atom in sample formulas.







