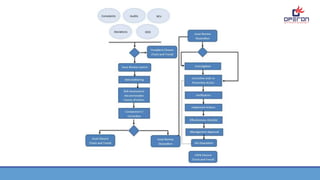
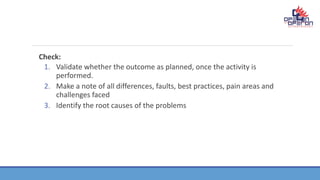
CAPA (Corrective and Preventive Action) is a quality management system used in the pharmaceutical and medical device industries to continuously improve processes, procedures, and the organization. CAPA involves corrective actions to address existing issues and preventive actions to prevent potential problems. The CAPA process follows the PDCA (Plan-Do-Check-Act) cycle. PDCA involves planning improvements, implementing them, checking results, and acting to correct or improve further. The goal of CAPA and PDCA is continual improvement through systematically addressing problems, identifying root causes, and verifying that issues have been resolved.