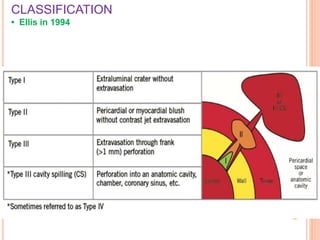
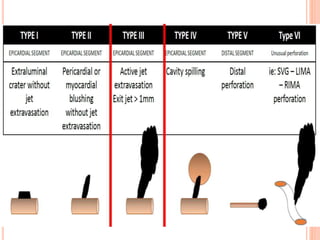
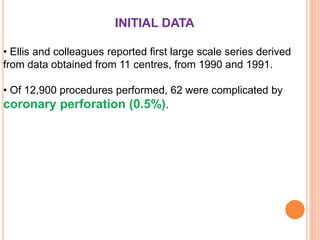
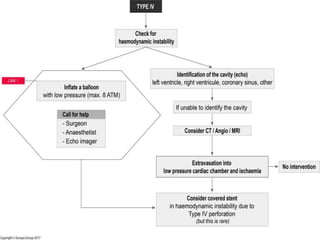
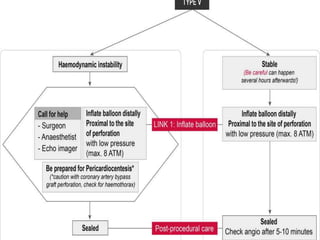
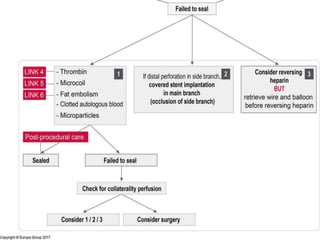
Coronary artery perforation is a serious complication of percutaneous coronary intervention that can lead to tamponade, shock, and death if not promptly recognized and treated. The summary describes:
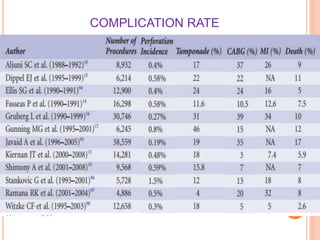
1) Coronary artery perforation occurs in 0.2-0.9% of PCIs and presents a challenge in balancing revascularization and risk of bleeding.
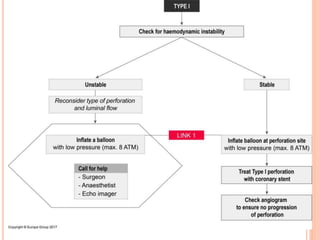
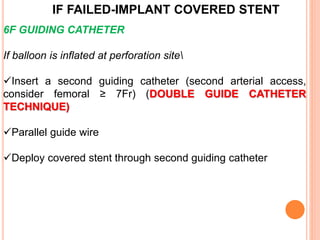
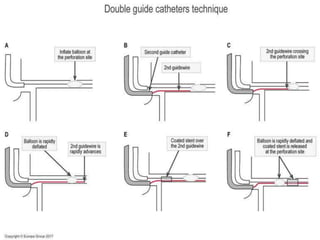
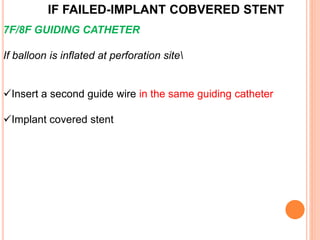
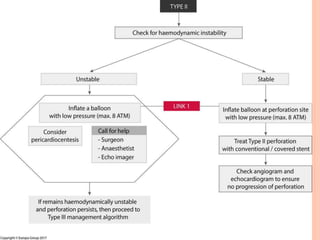
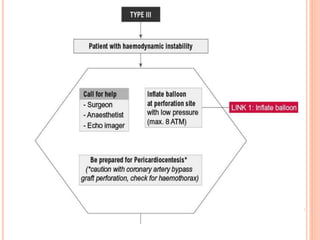
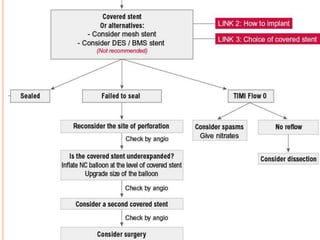
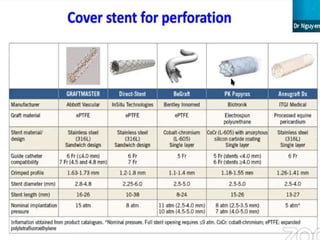
2) Initial treatment involves inflating a balloon at the perforation site to seal it off, followed by deployment of a covered stent if needed.
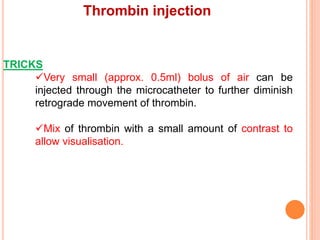
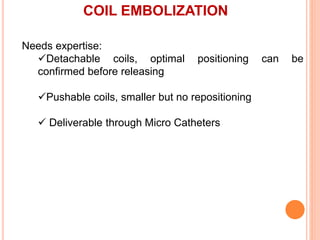
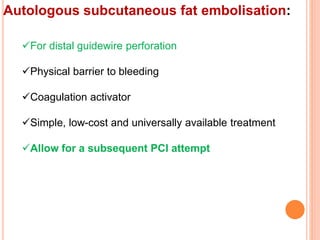
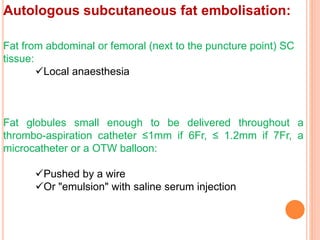
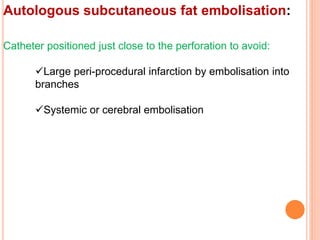
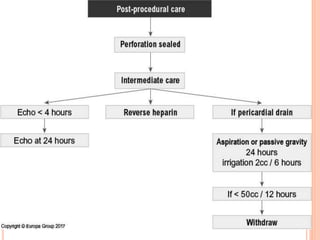
3) For distal perforations, thrombin, coil, or fat embolization may be used to seal the perforation. Close monitoring is needed for at least 24 hours due to the risk of