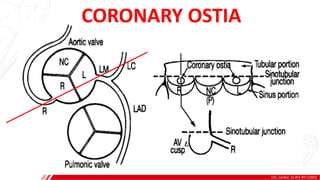
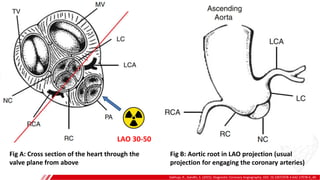
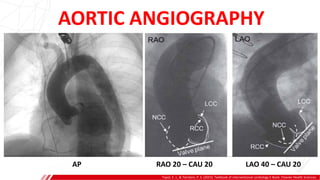
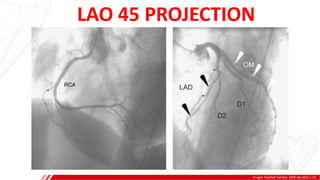
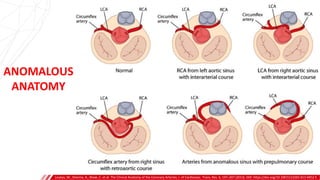
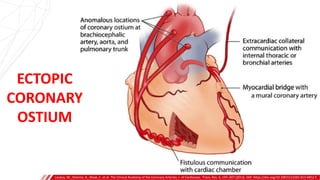
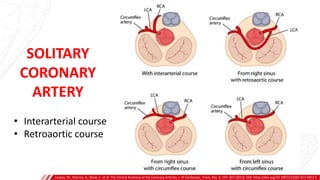
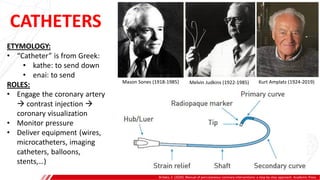
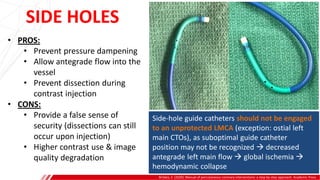
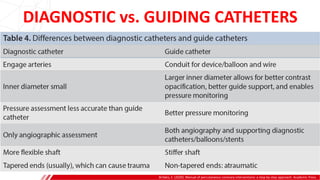
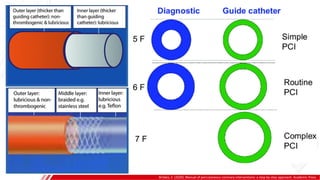
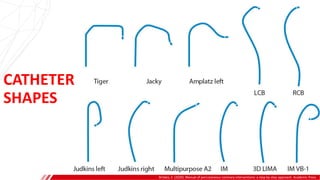
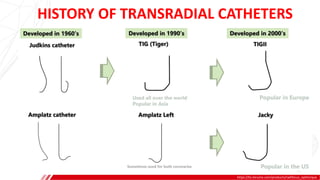
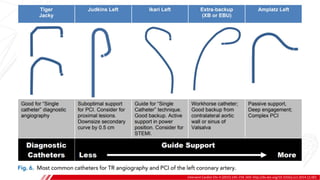
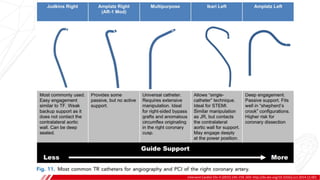
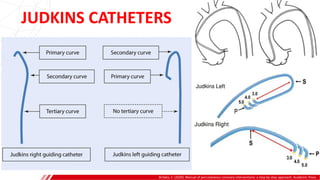
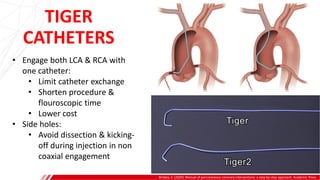
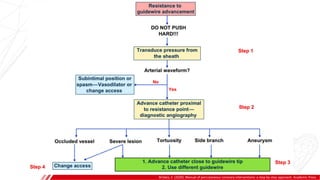
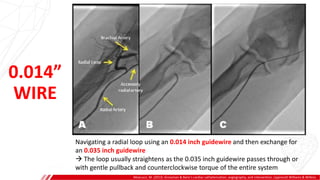
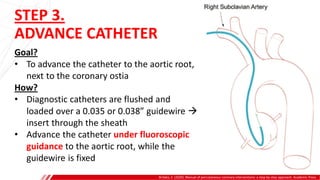
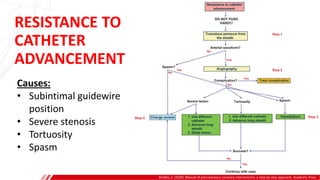
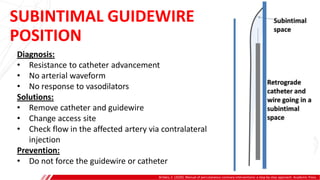
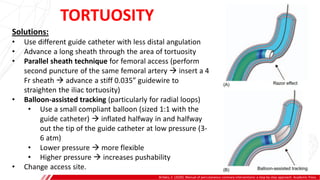
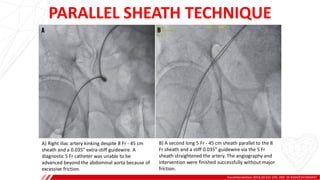
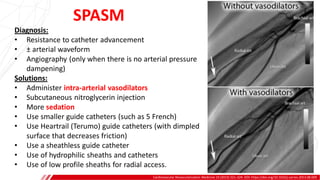
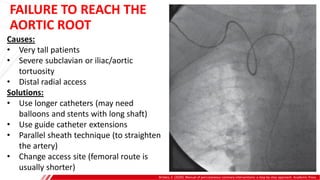
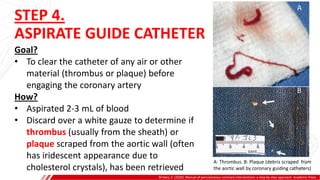
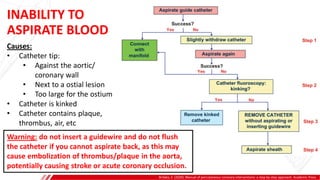
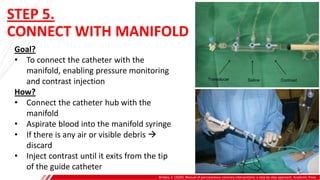
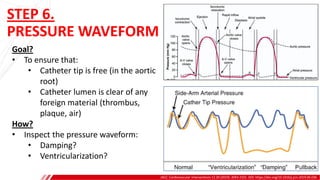
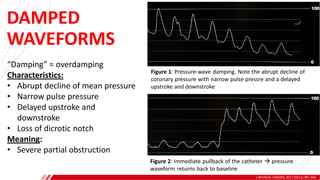
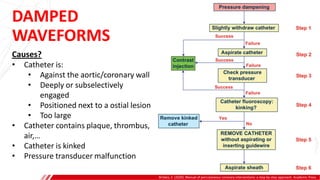
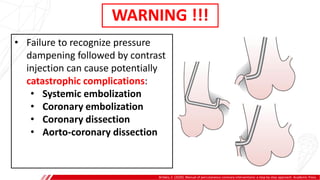
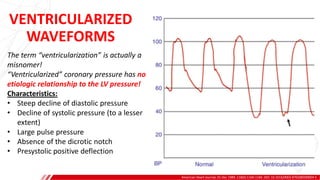
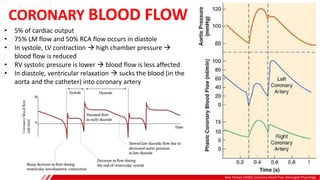
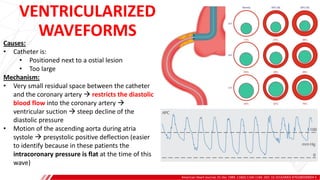
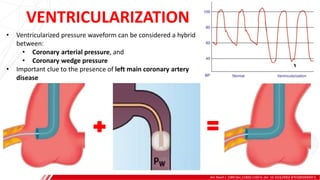
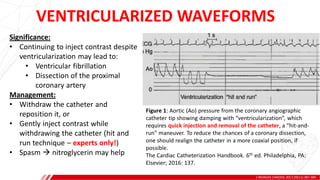
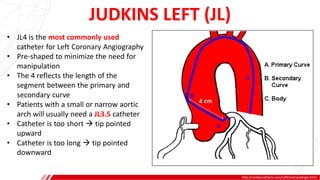
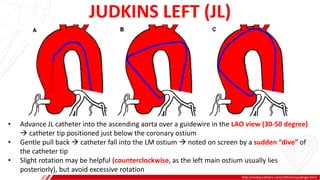
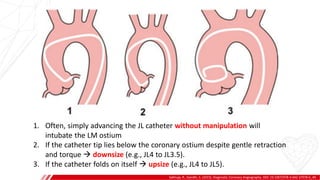
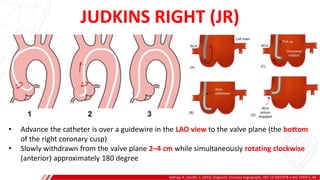
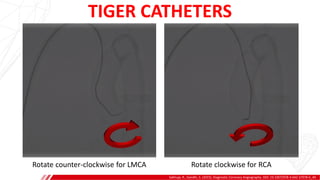
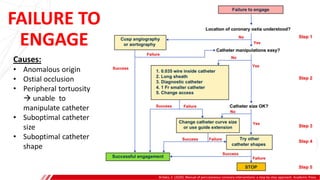
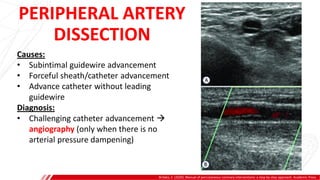
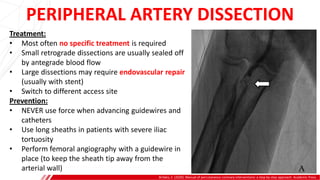
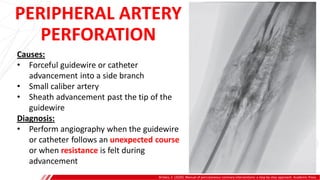
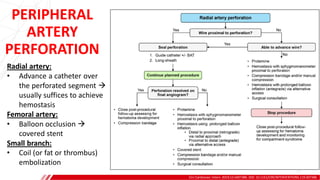
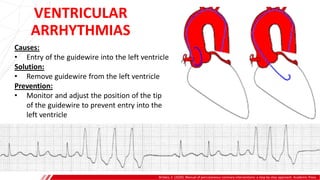
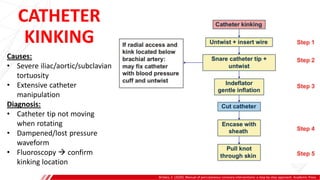
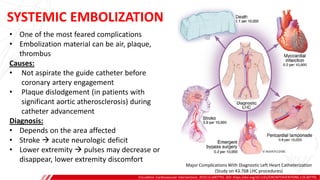
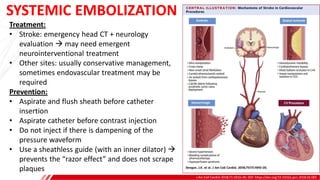
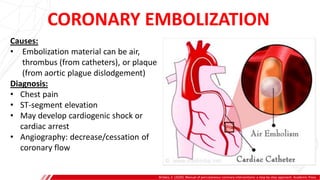
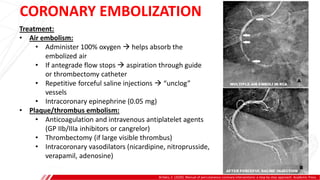
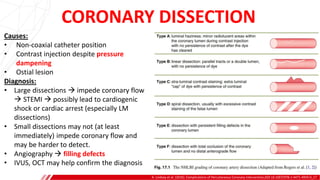
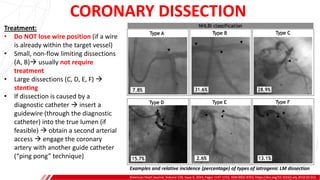
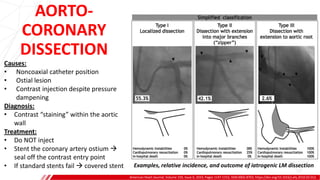
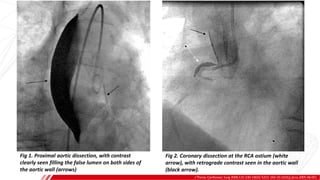
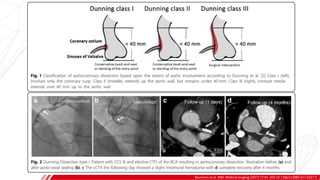
The document outlines the anatomy and techniques related to coronary engagement in interventional cardiology, focusing on catheter selection, procedure steps, and potential complications. It emphasizes the importance of proper catheter positioning, pressure monitoring, and strategies to address challenges like resistance or spasm during coronary procedures. Additionally, it provides historical context on catheter development and notes critical considerations for ensuring procedural success and patient safety.