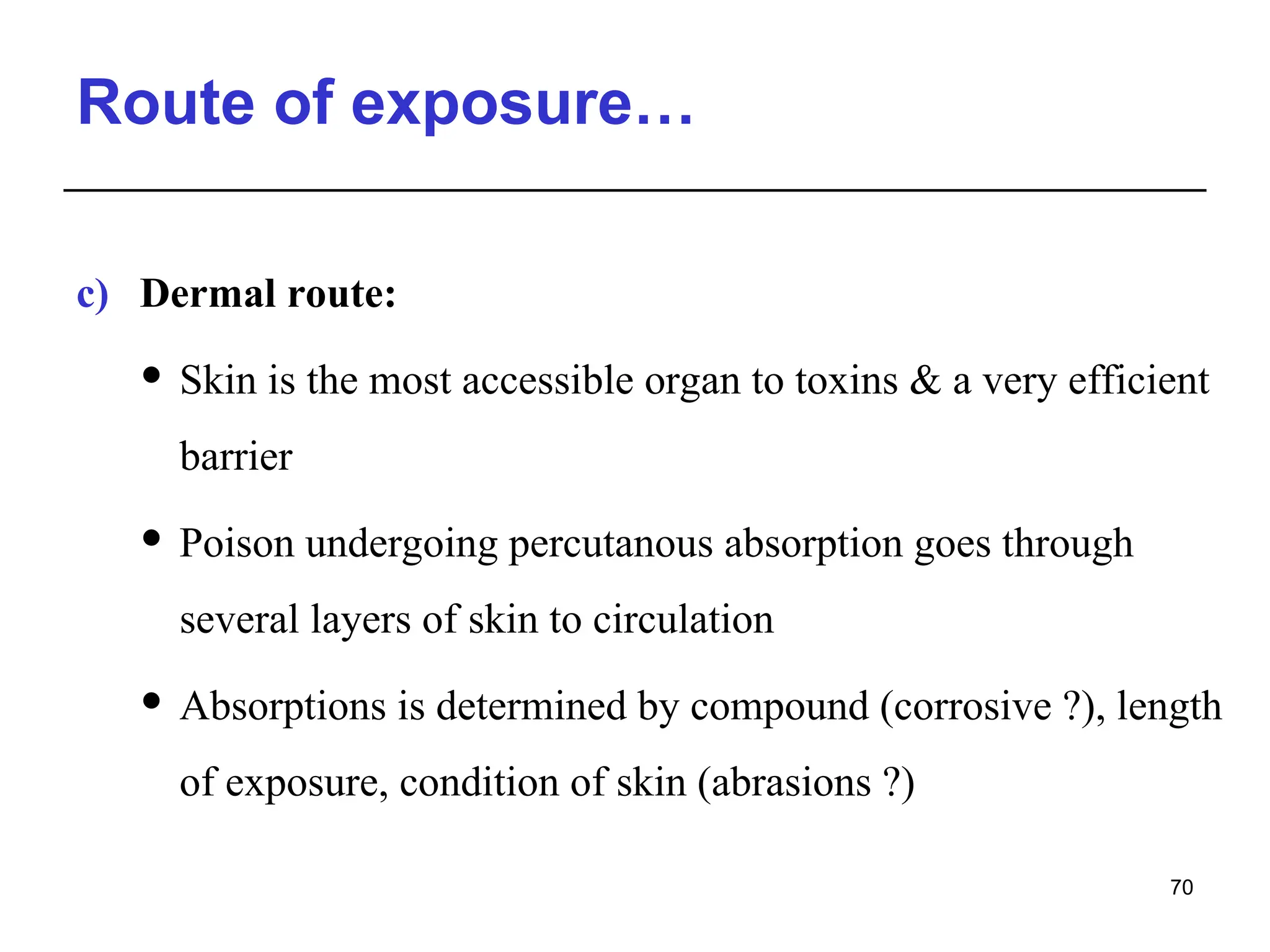
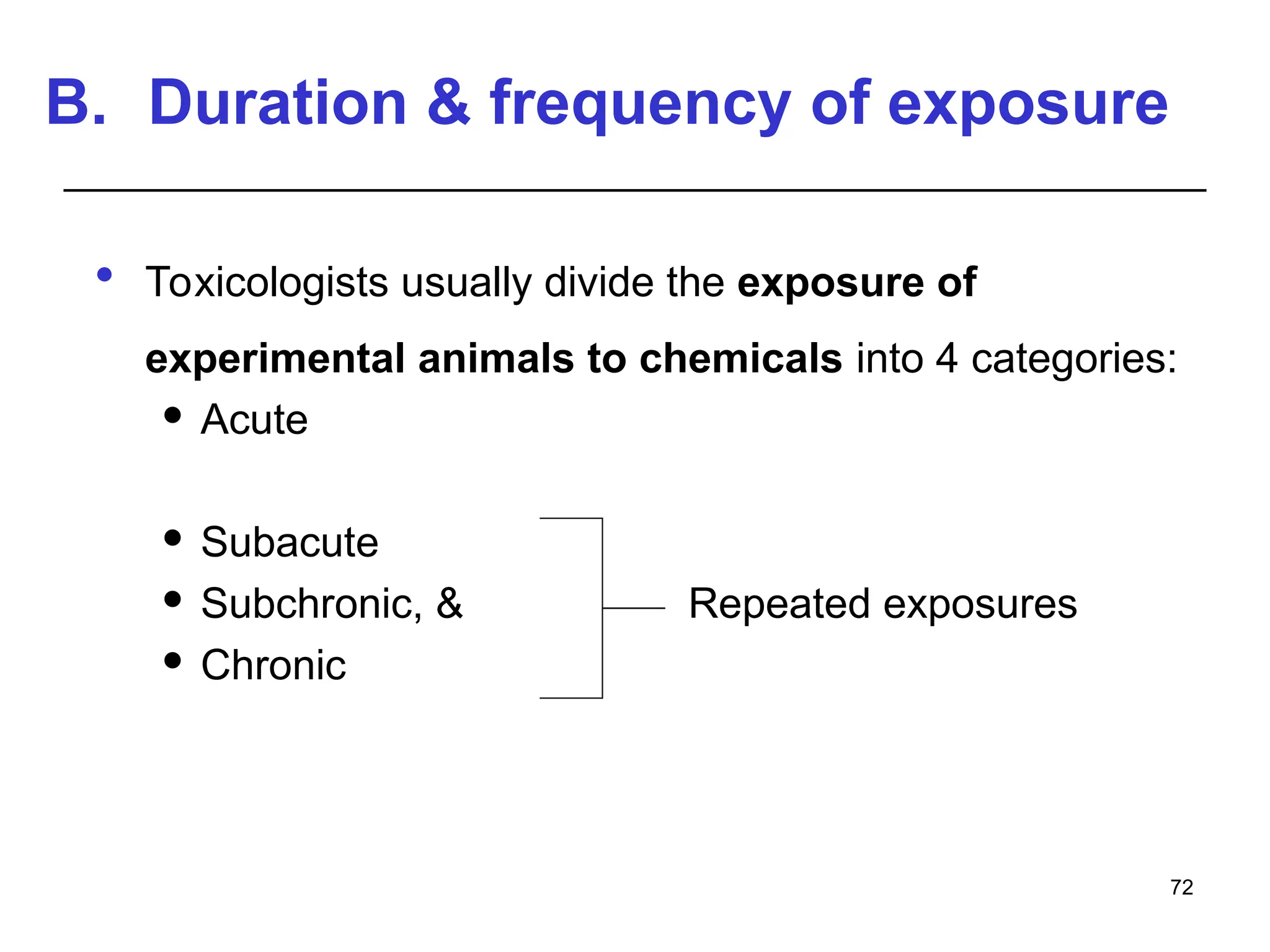
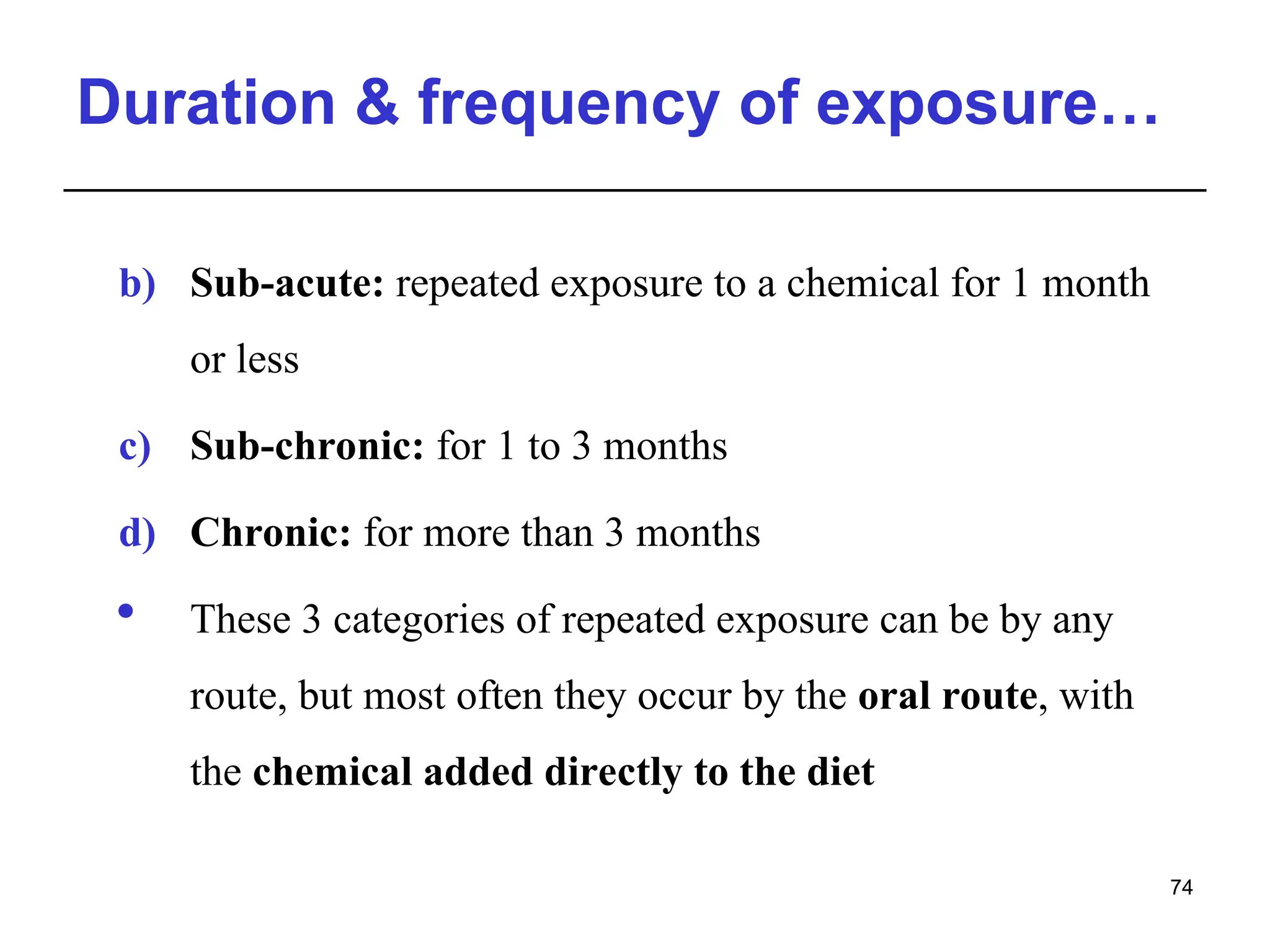
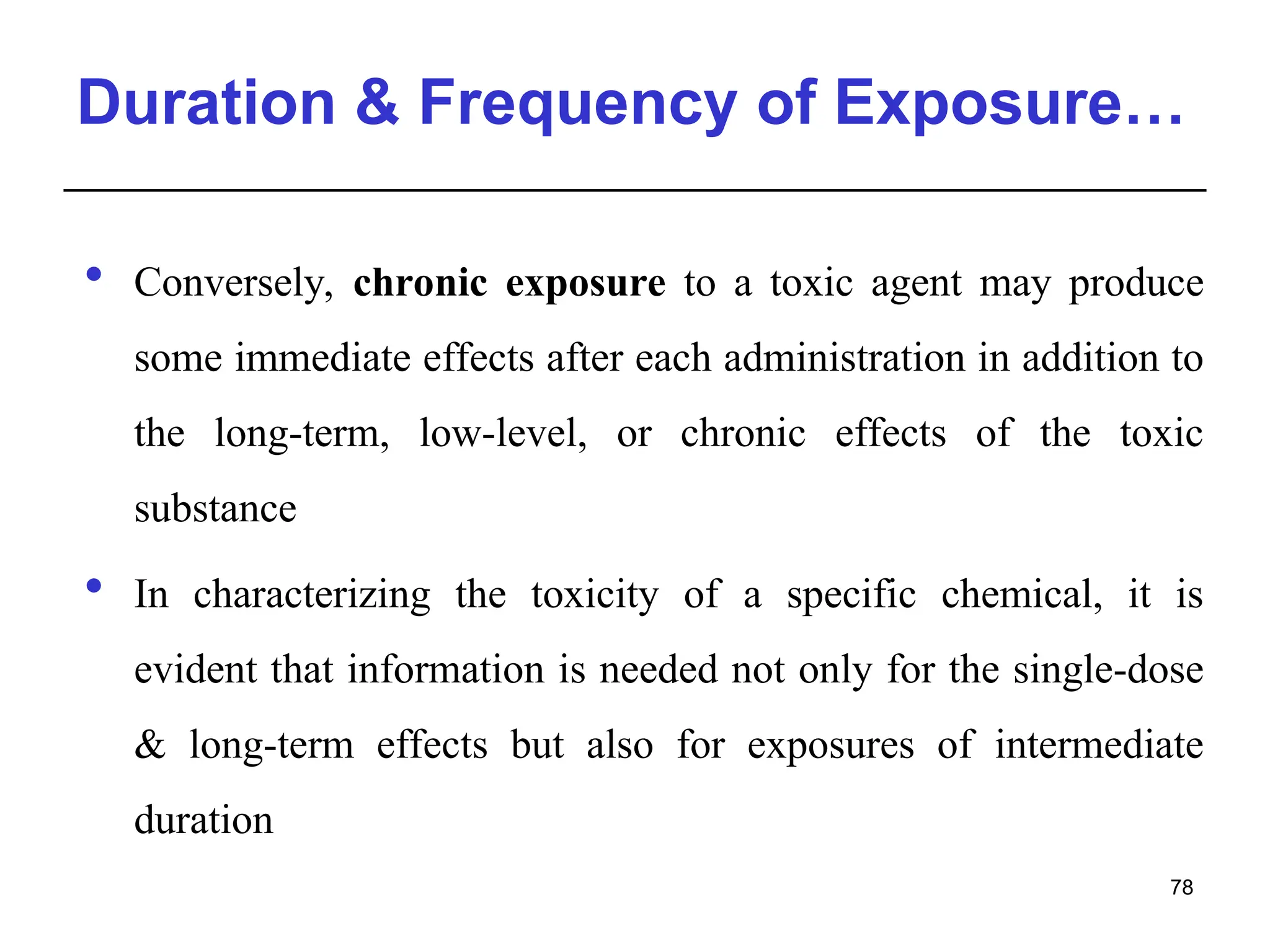
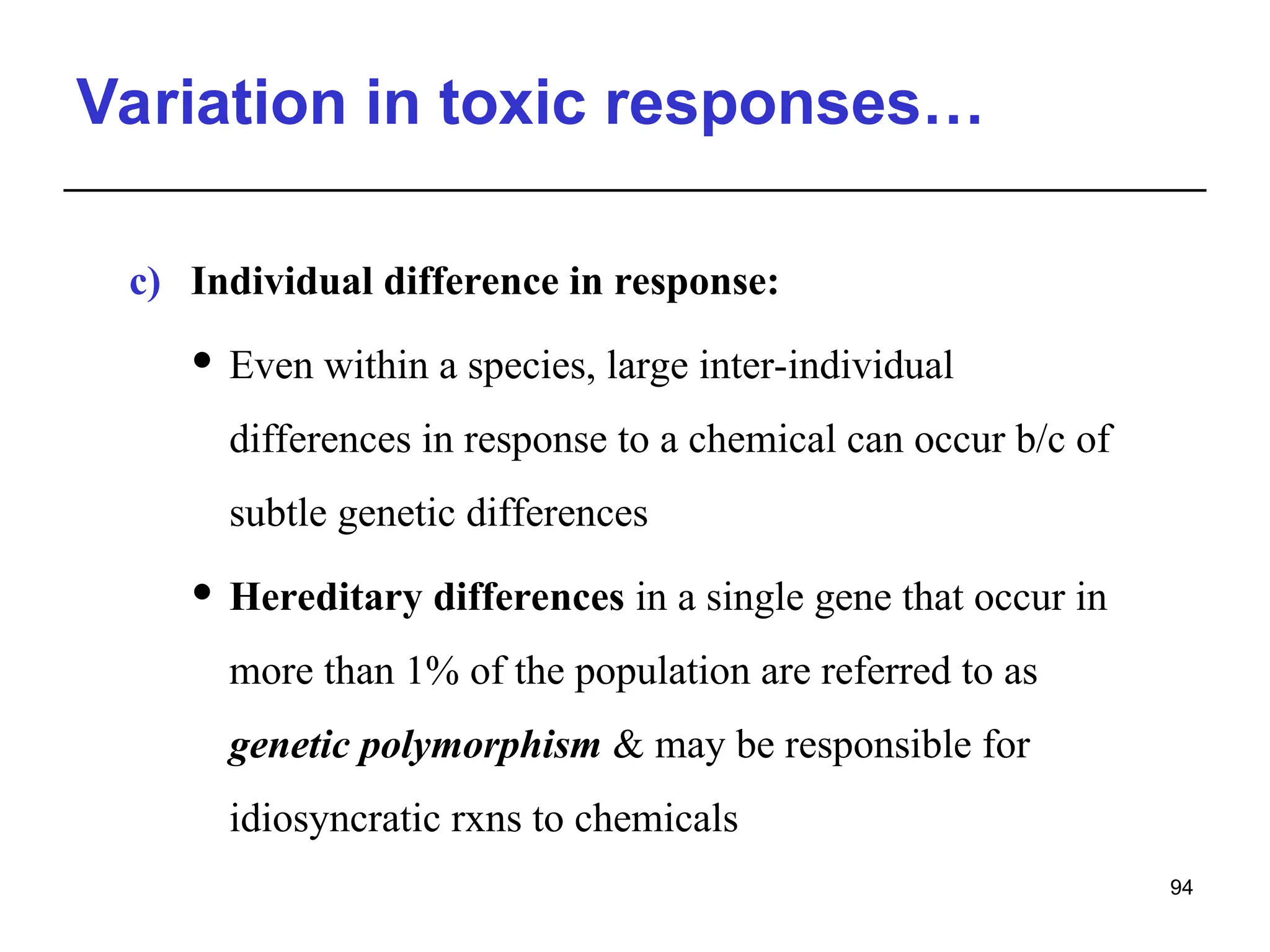
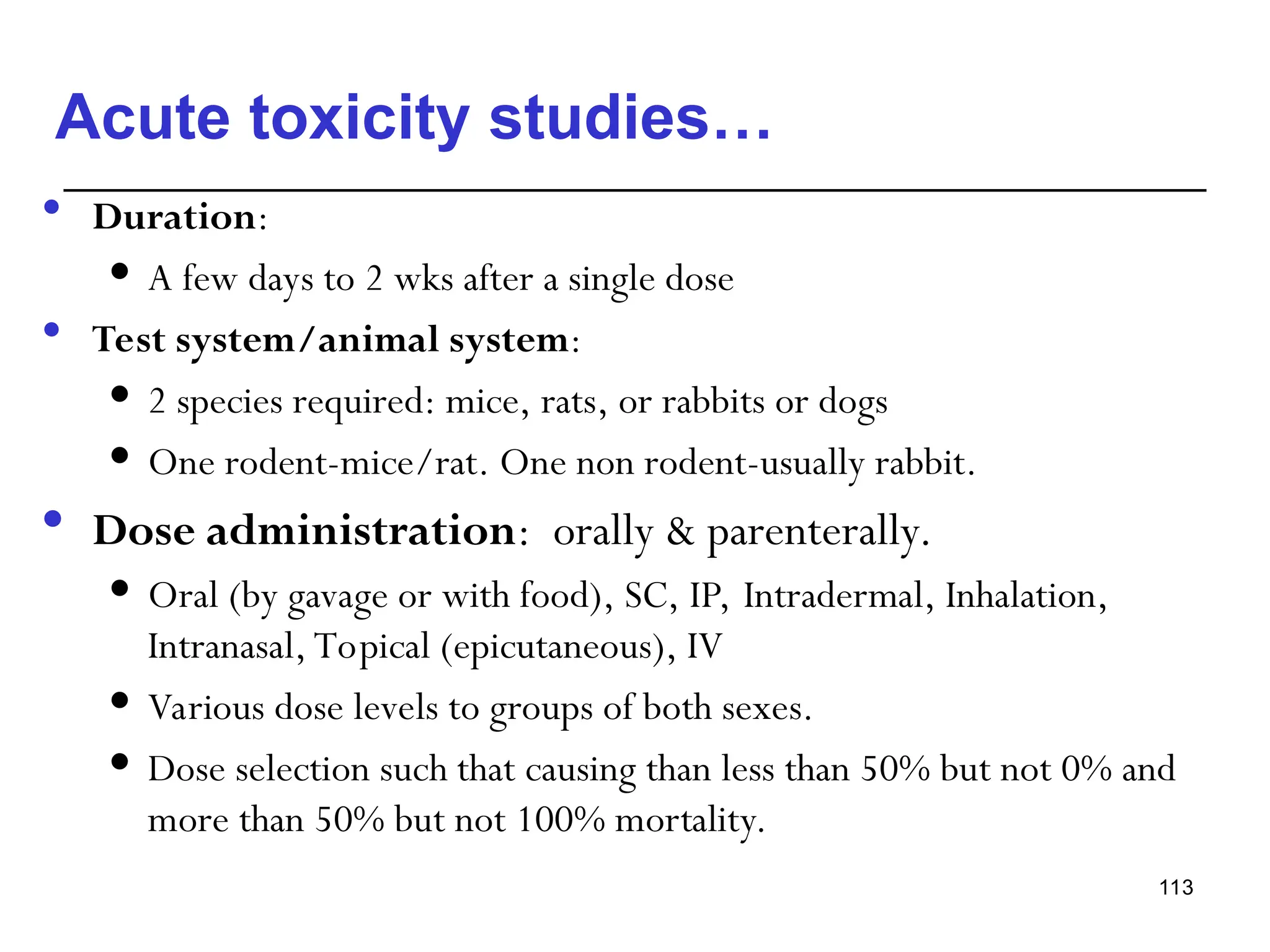
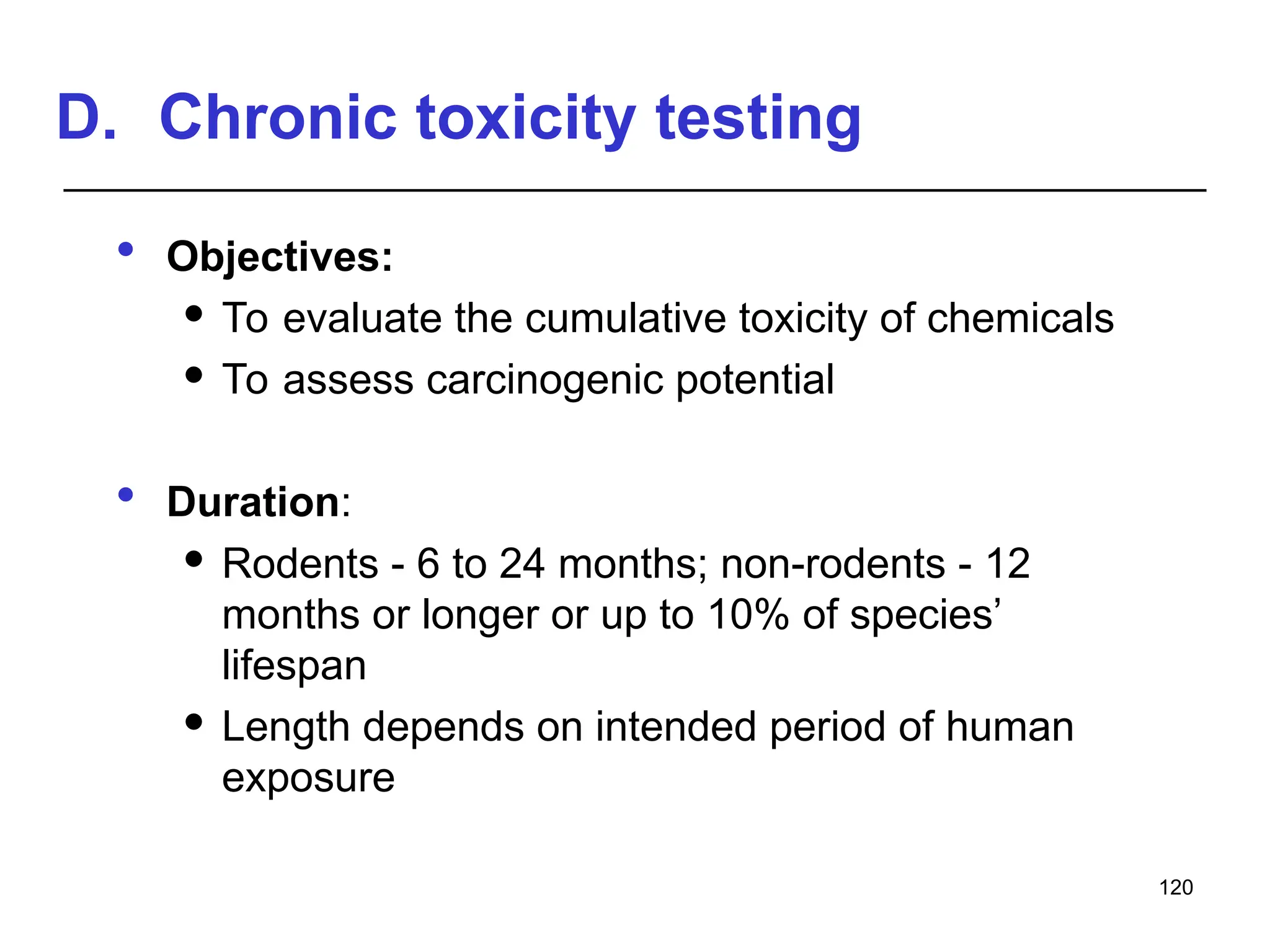
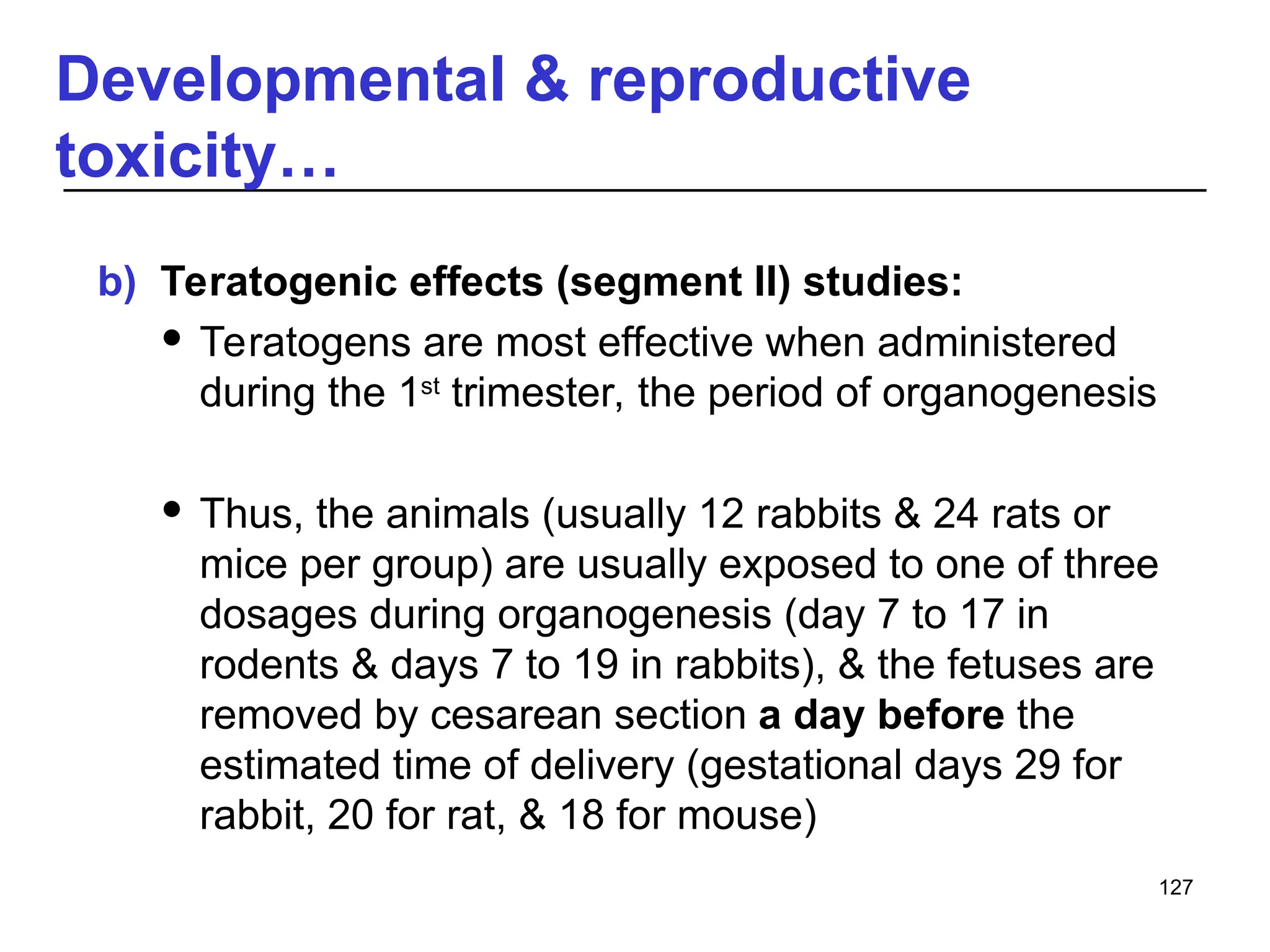
This document provides an overview of toxicology, defining it as the study of the adverse effects of various agents on living organisms and the environment, along with its history and importance in different areas such as clinical, environmental, and regulatory toxicology. It discusses the classification of toxic agents, their effects, and the factors influencing toxicity, such as dose and exposure. The document emphasizes the significance of toxicology in medicine, agriculture, and environmental safety.




































![I. Spectrum of toxic dose
Paracelsus (1493–1541), phrased this well when
he noted:
“What is there that is not poison? All things are
poison & nothing [is] without poison. solely the
dose determines that a thing is not a poison”
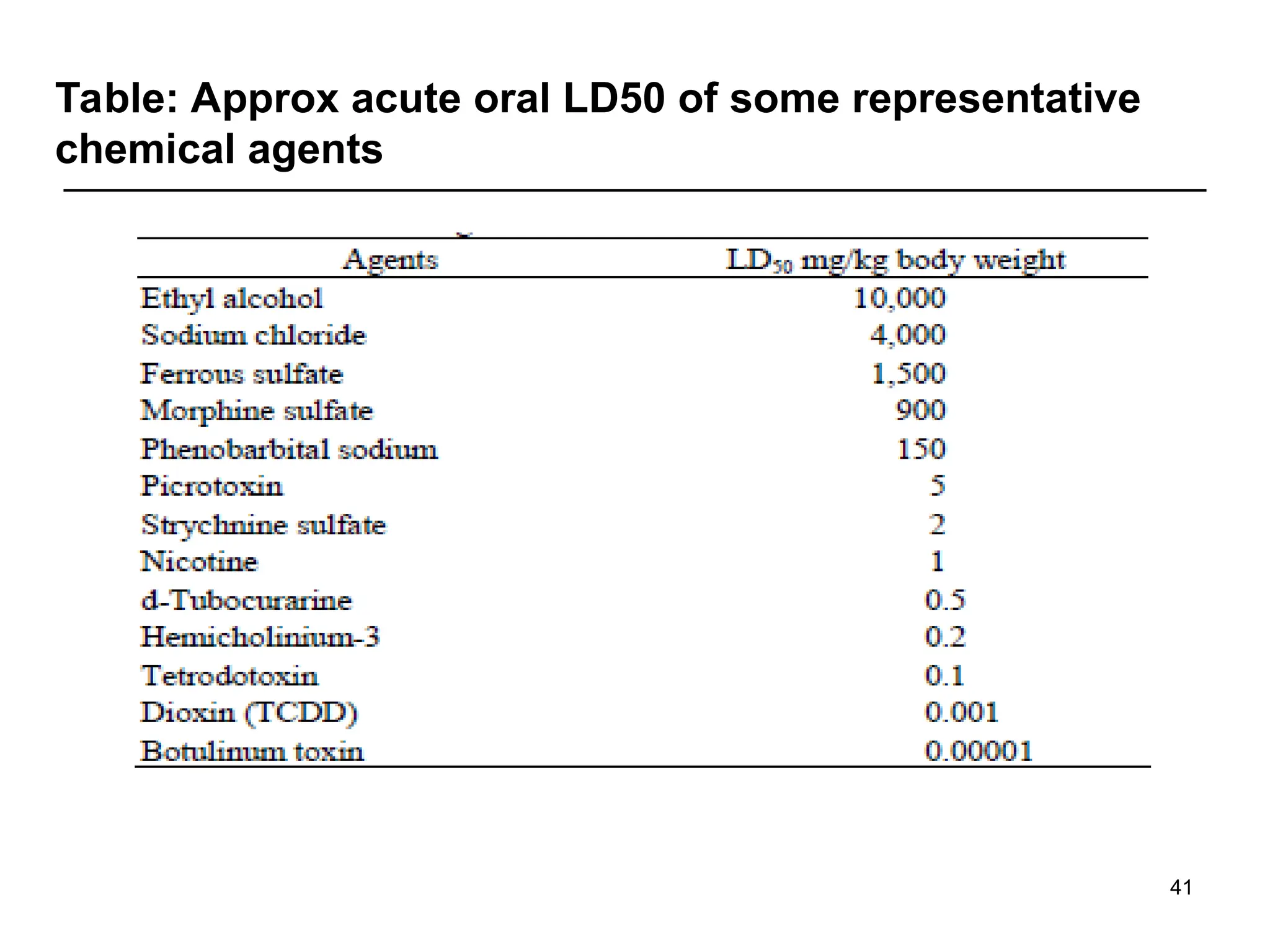
E.g. in mouth LD50 of water = 44ml/kg, saline
=68ml/kg
Even very toxic substances may not be toxic at
low conc
• E.g Botlinium toxin used as a cosmetic
38](https://image.slidesharecdn.com/chapter12-250207171954-90b0507a/75/Chapter-1-2-ppt-38-2048.jpg)



![Classification of toxic agents…
Toxin: refers to toxic substances that are
produced by biological systems such as plants,
animals, fungi or bacteria
E.g. Zeralanone, produced by a mold
Toxicant: used in speaking of toxic substances
that are produced by or are a by-product of
anthropogenic (human-made) activities
E.g. Dioxin [2,3,7,8-tetrachlorodibenzop- dioxin
(TCDD)], produced during the combustion of
certain chlorinated organic chemicals
44](https://image.slidesharecdn.com/chapter12-250207171954-90b0507a/75/Chapter-1-2-ppt-44-2048.jpg)