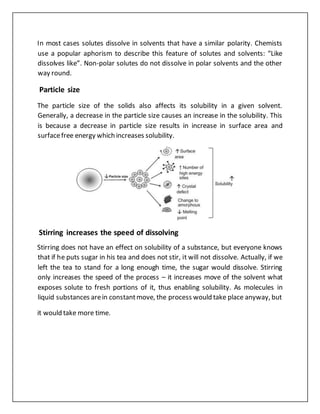
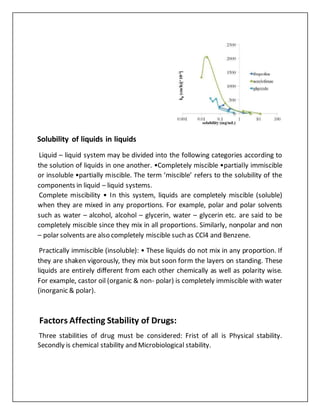
Several factors affect the solubility and stability of drugs. Solubility increases with temperature for most solvents but decreases for gases and some salts. Solubility also depends on polarity, with like dissolving like. Smaller particle size increases surface area and solubility. Stirring speeds dissolution but does not affect solubility. Intermolecular forces between solute and solvent impact solubility. Drug solubility is also affected by pH and ability to ionize. Stability is influenced by physical changes like crystallization, chemical reactions like hydrolysis, and microbiological contamination. Light, packaging materials, and temperature can also impact drug stability over time.