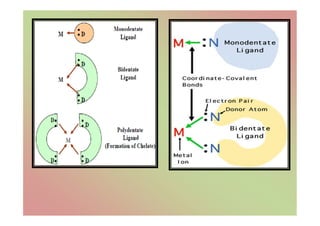
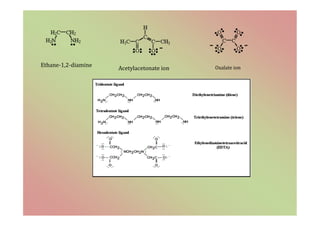
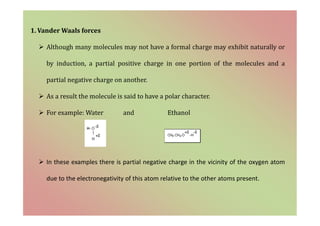
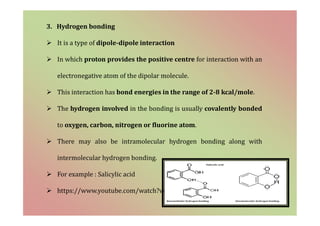
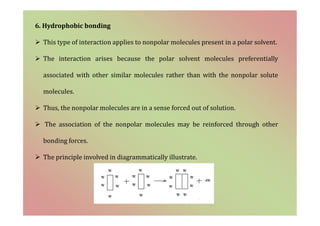
The document discusses the different types of bonding forces that can be operative in complex formation, including van der Waals forces, hydrogen bonding, charge transfer interactions, ion pairing, and hydrophobic interactions. It provides examples of each type of interaction and how they contribute to complex stability. Monodentate and polydentate ligands are described as well as their role in chelation and forming stable metal complexes.