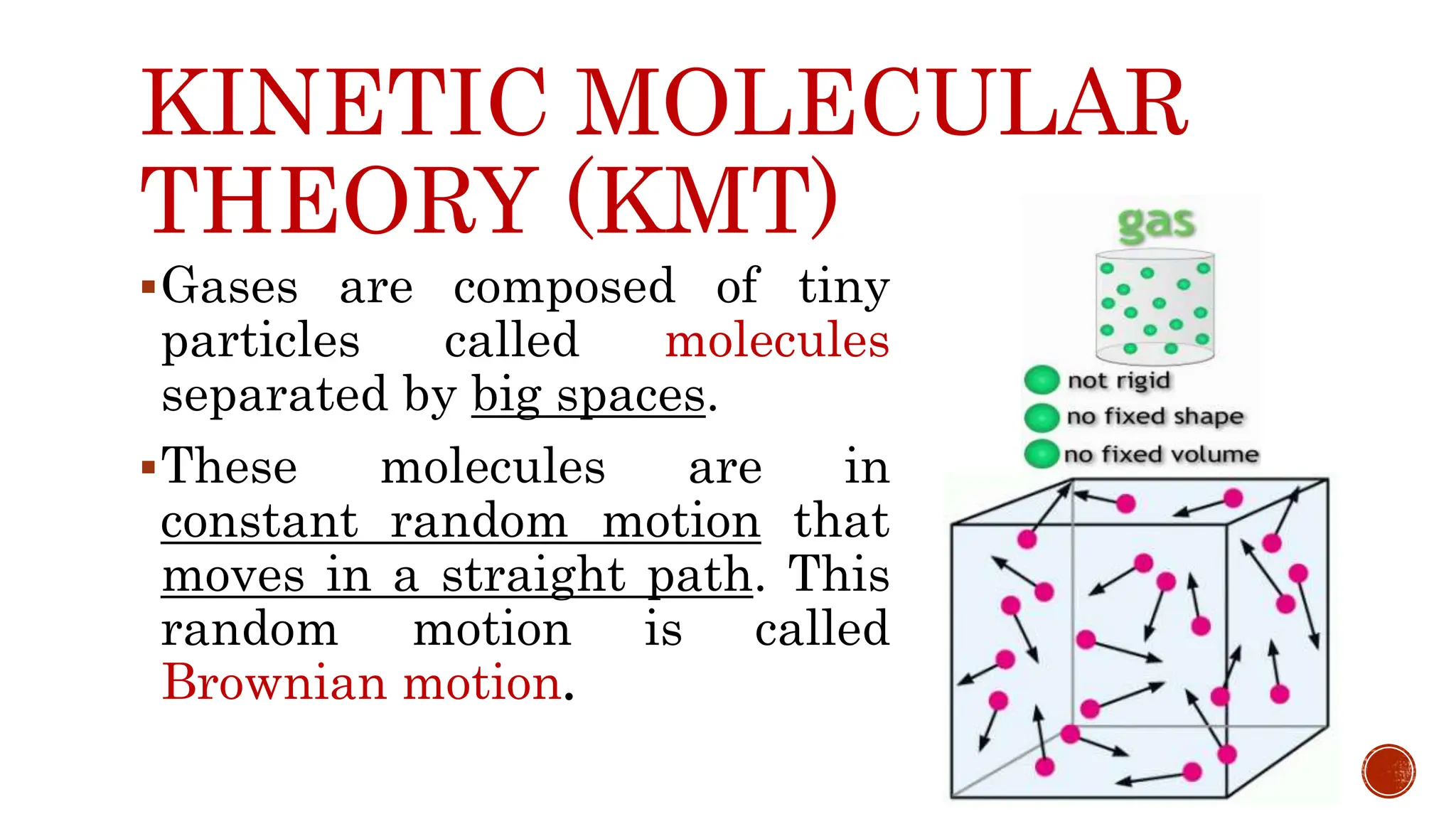
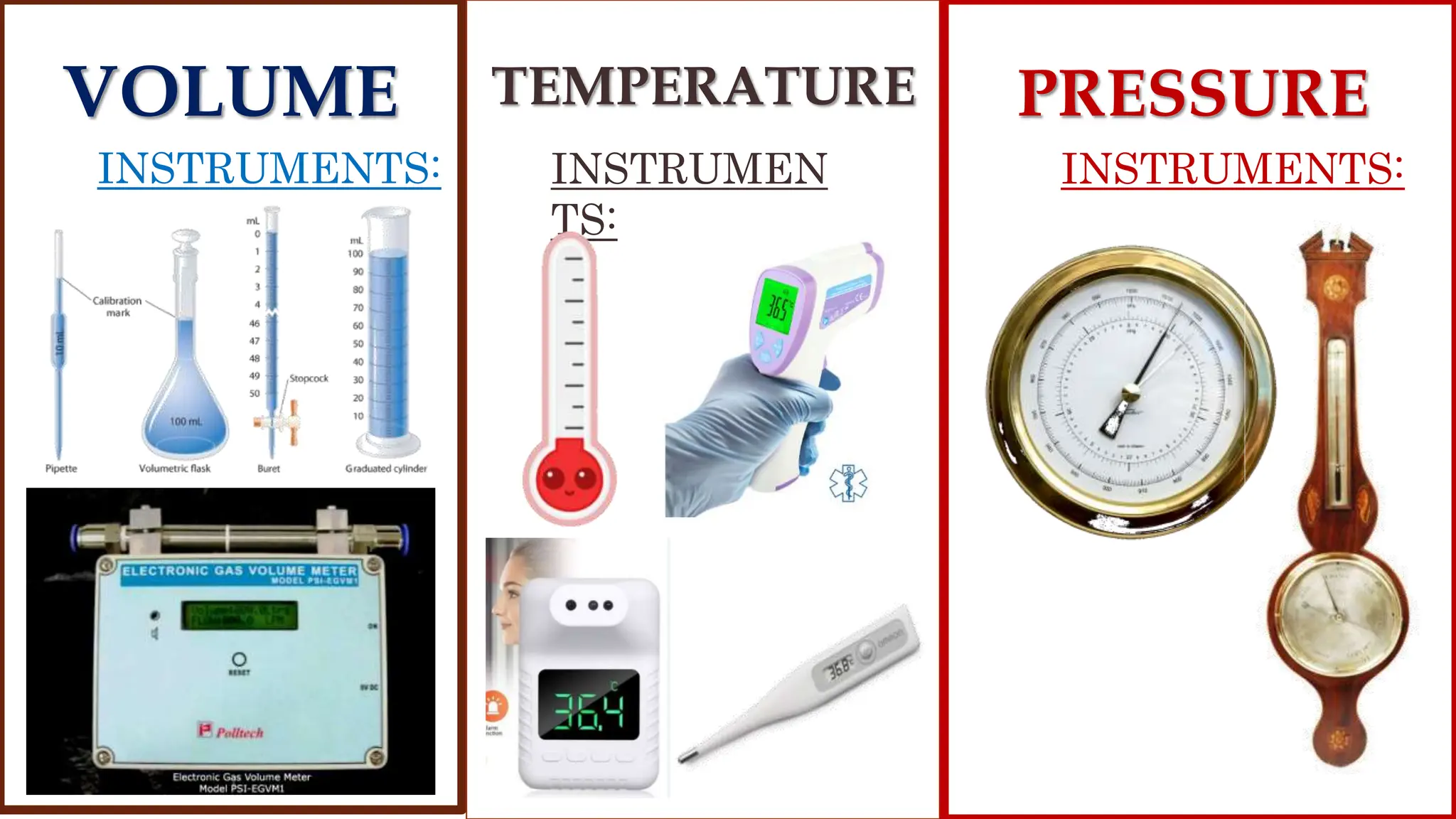
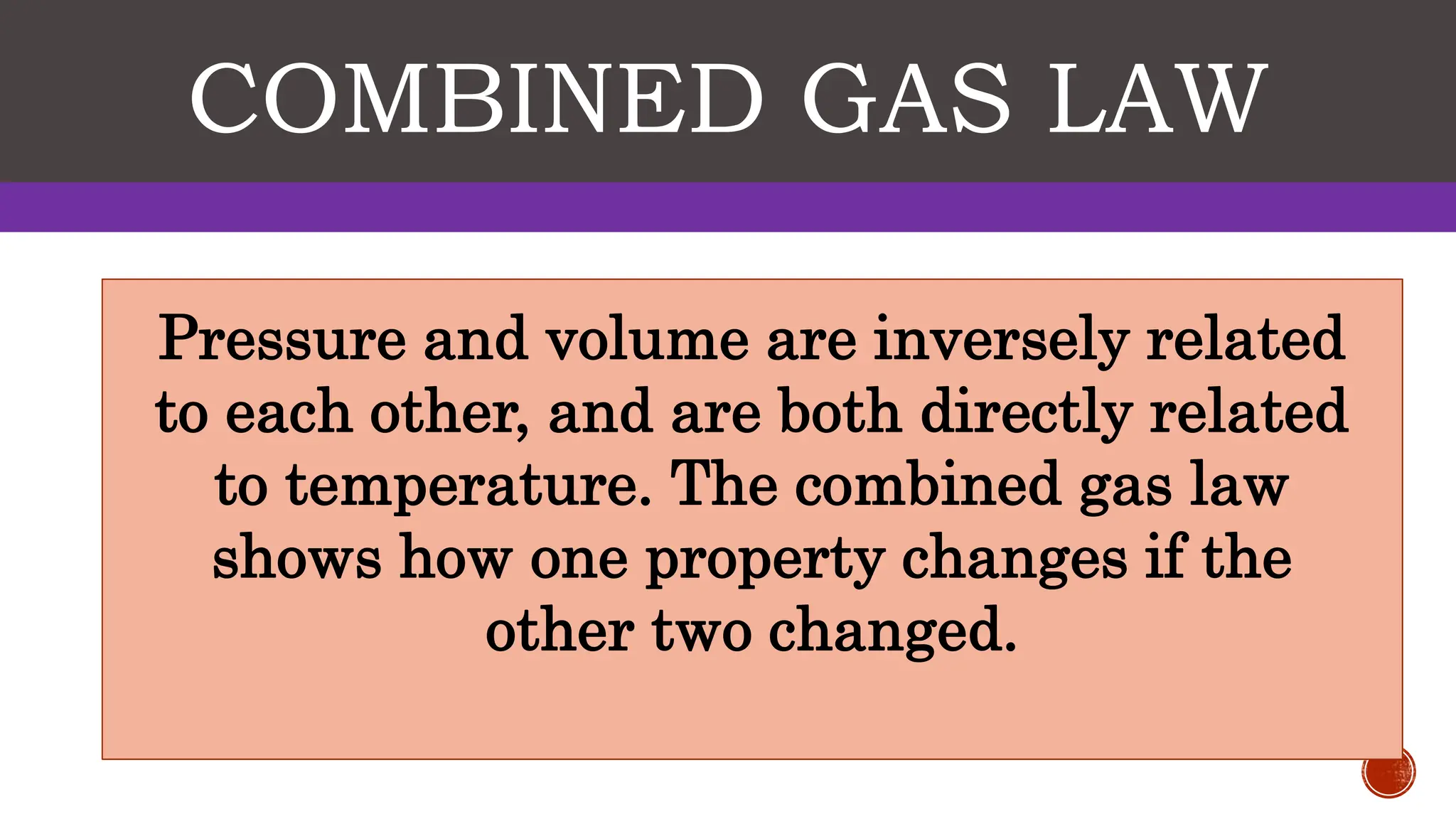
The kinetic molecular theory explains gas behavior through the motion of molecules, which occupy space, have mass, and display properties like pressure, temperature, and volume. Key gas laws including Boyle's Law, Charles' Law, and Gay-Lussac's Law describe the relationships between these properties, revealing how changes in one affect the others. The combined gas law incorporates pressure, volume, and temperature, highlighting its practical applications in everyday scenarios such as cloud formation and refrigeration.