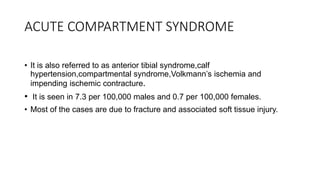
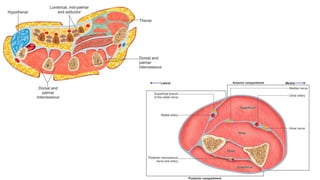
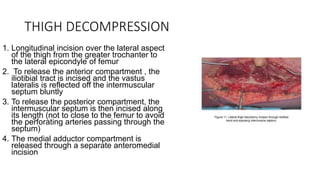
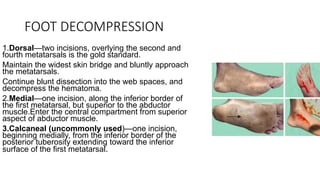
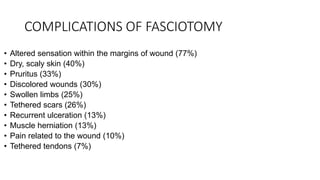
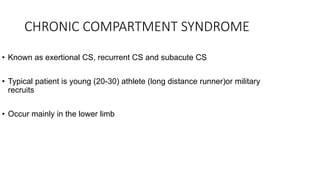
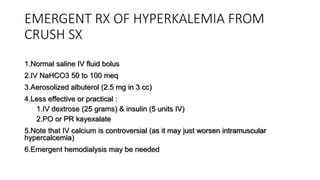
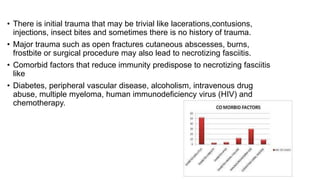
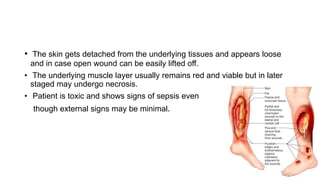
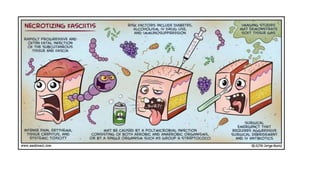
- Crush injury syndrome, also known as compartment syndrome, occurs when increased pressure within an osteofascial compartment reduces blood flow, resulting in tissue damage.
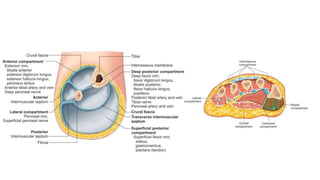
- It was first described in 1881 and various studies since then have identified the anatomy of compartments and improved understanding of the condition.
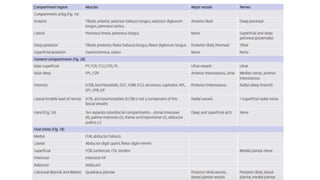
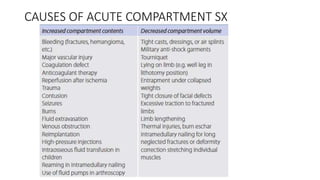
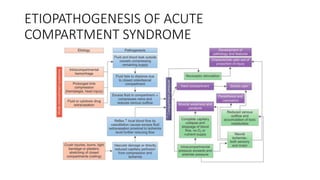
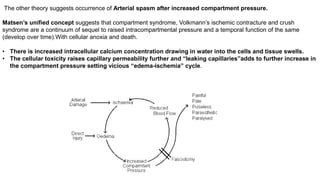
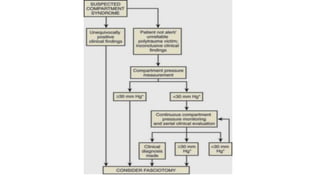
- Compartment syndrome is most often caused by fractures but can result from tight bandages, embolisms, or arterial issues. It develops when intracompartment pressure exceeds tissue perfusion pressure.
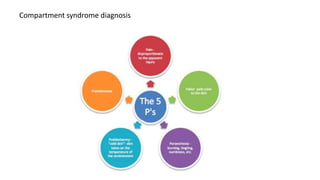
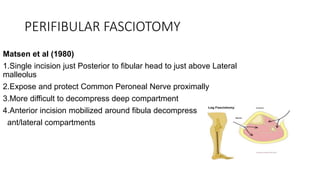
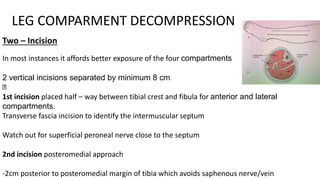
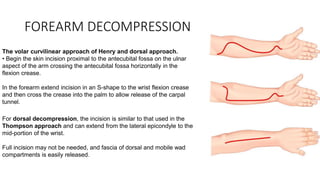
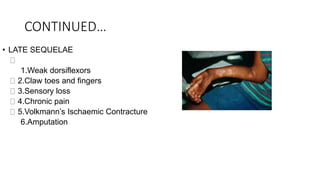
- Untreated, it leads to muscle and nerve damage within hours and contractures over days. Diagnosis is based on symptoms of pain, paresthesia, paralysis and objective measurement of compartment pressures. Early fasciotomy can prevent long