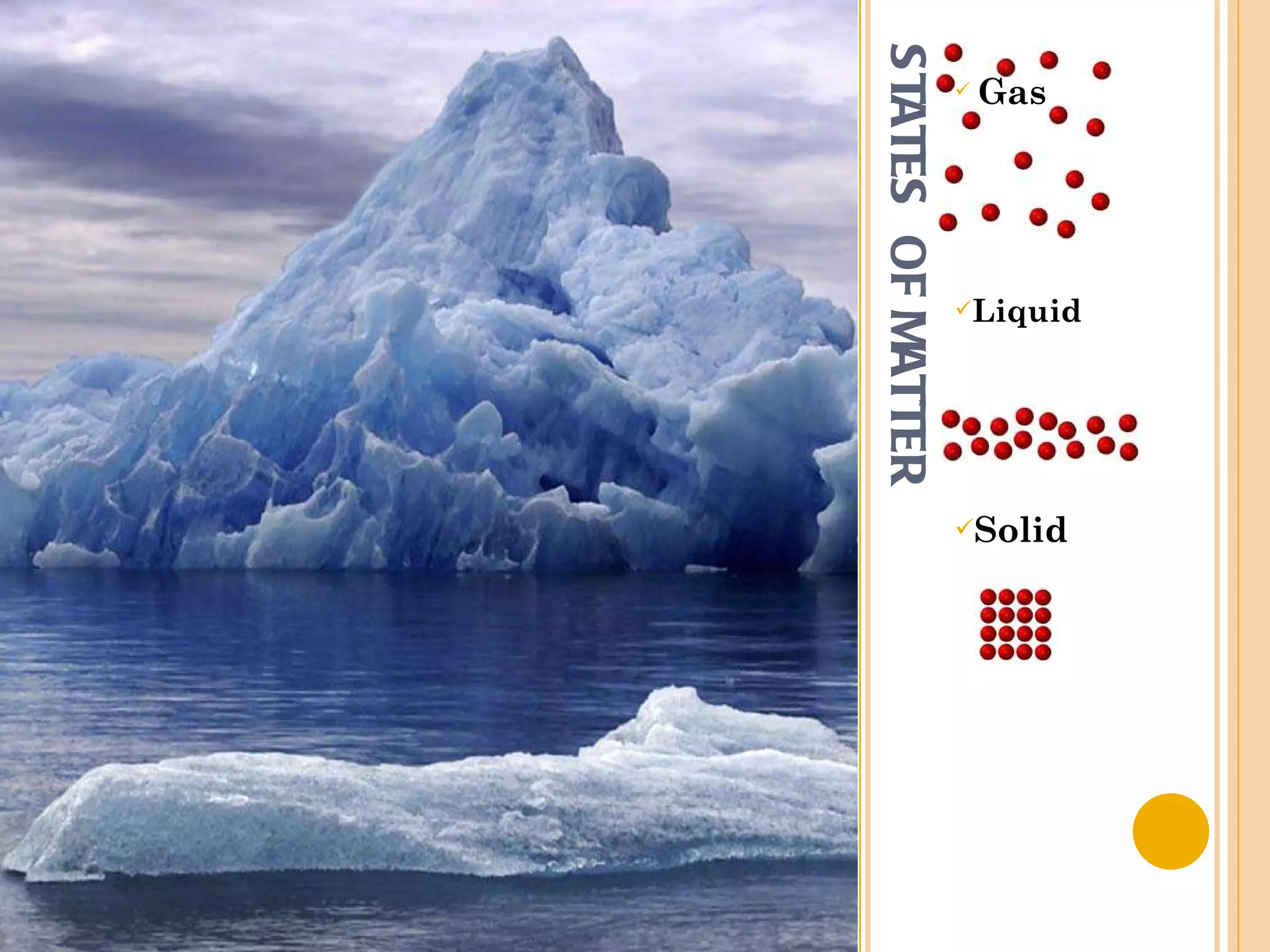
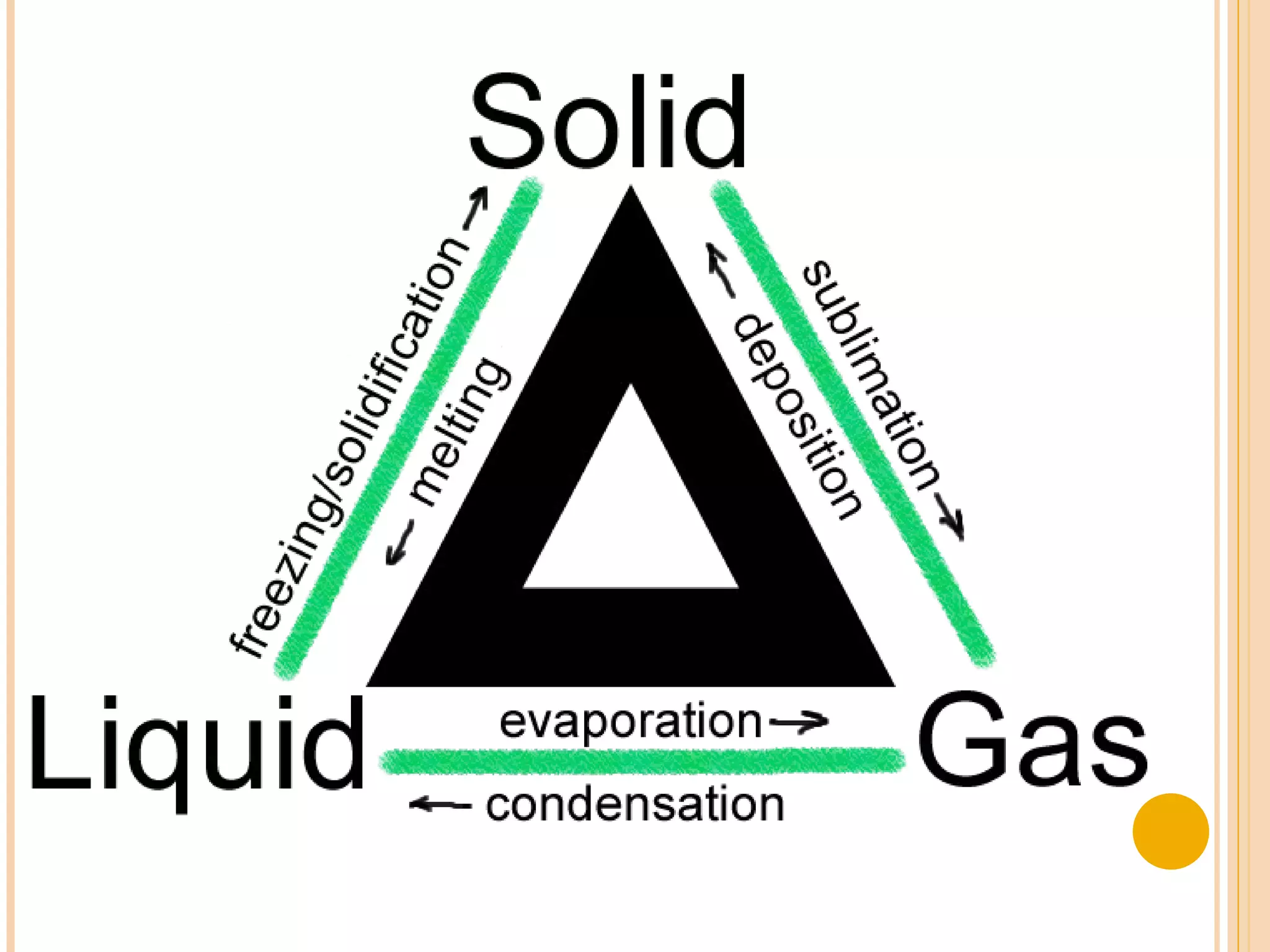
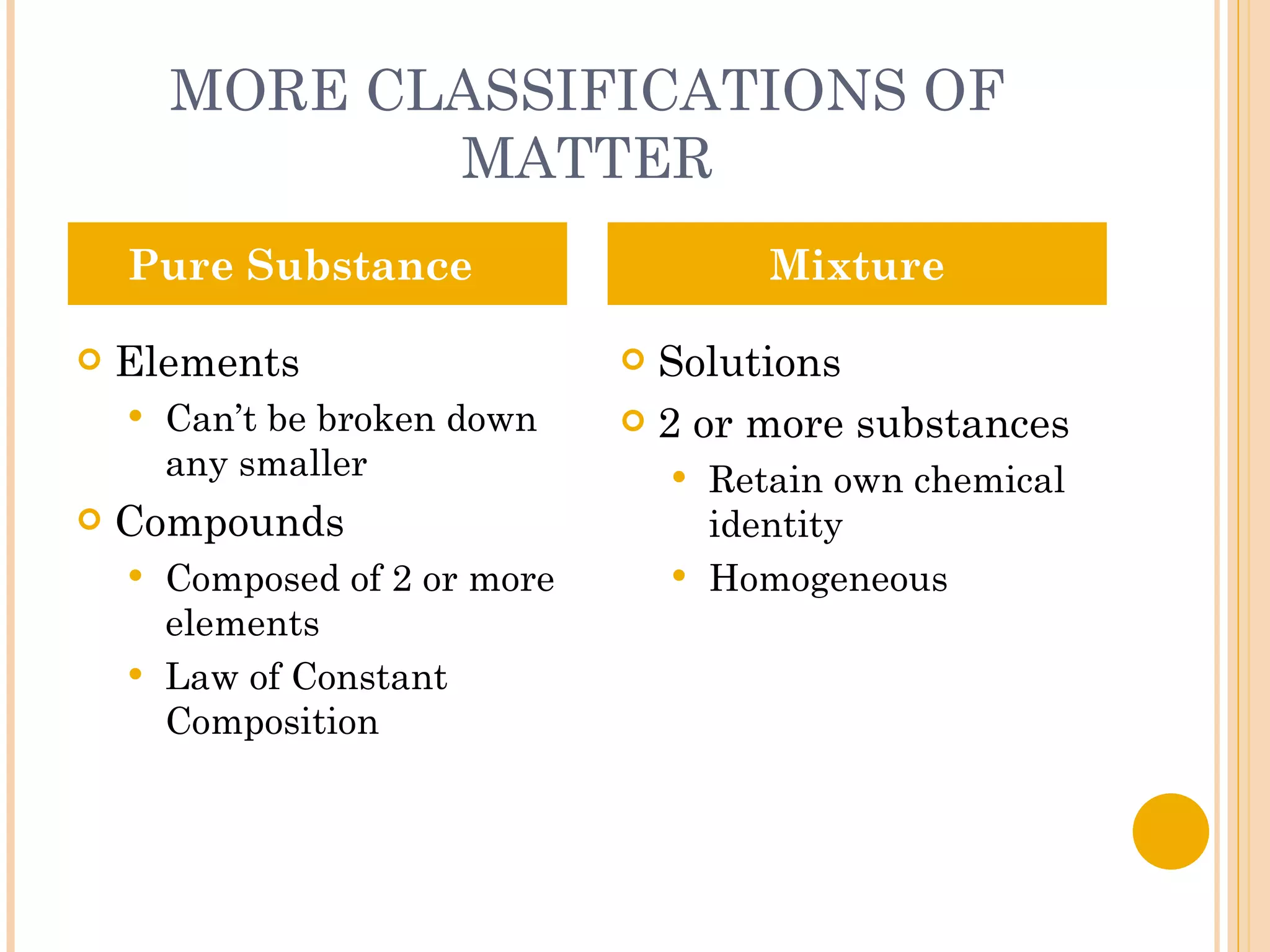
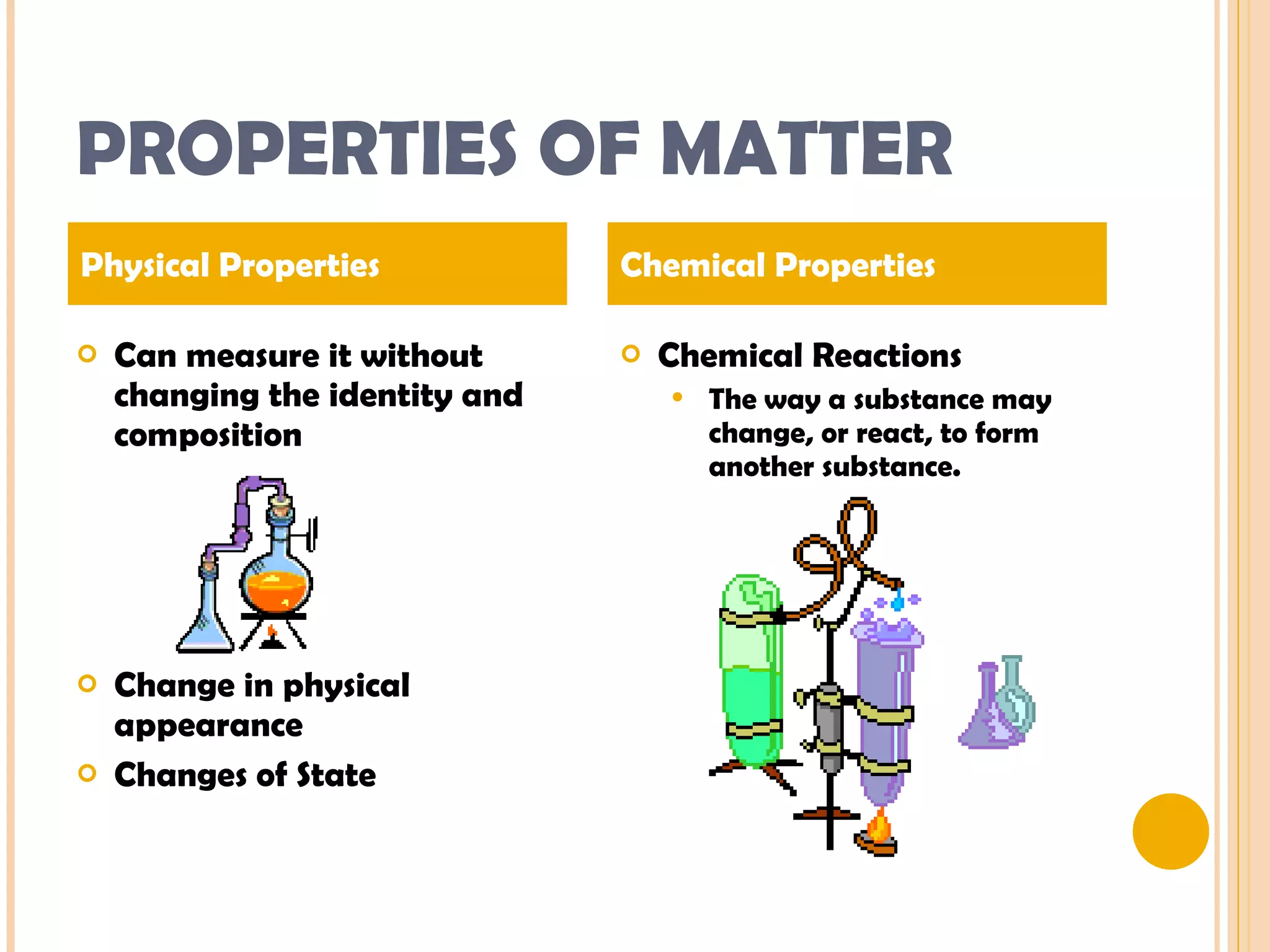
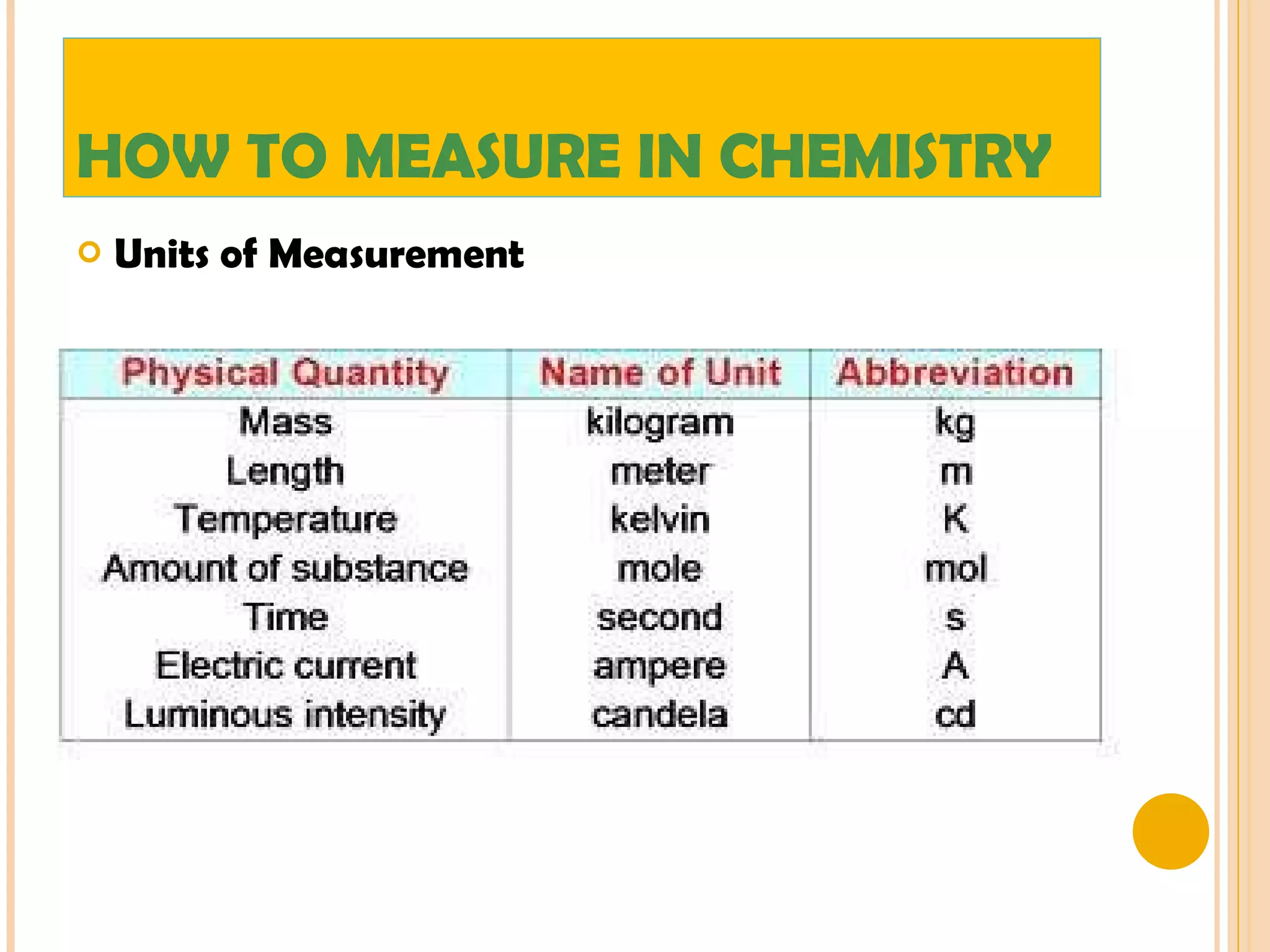
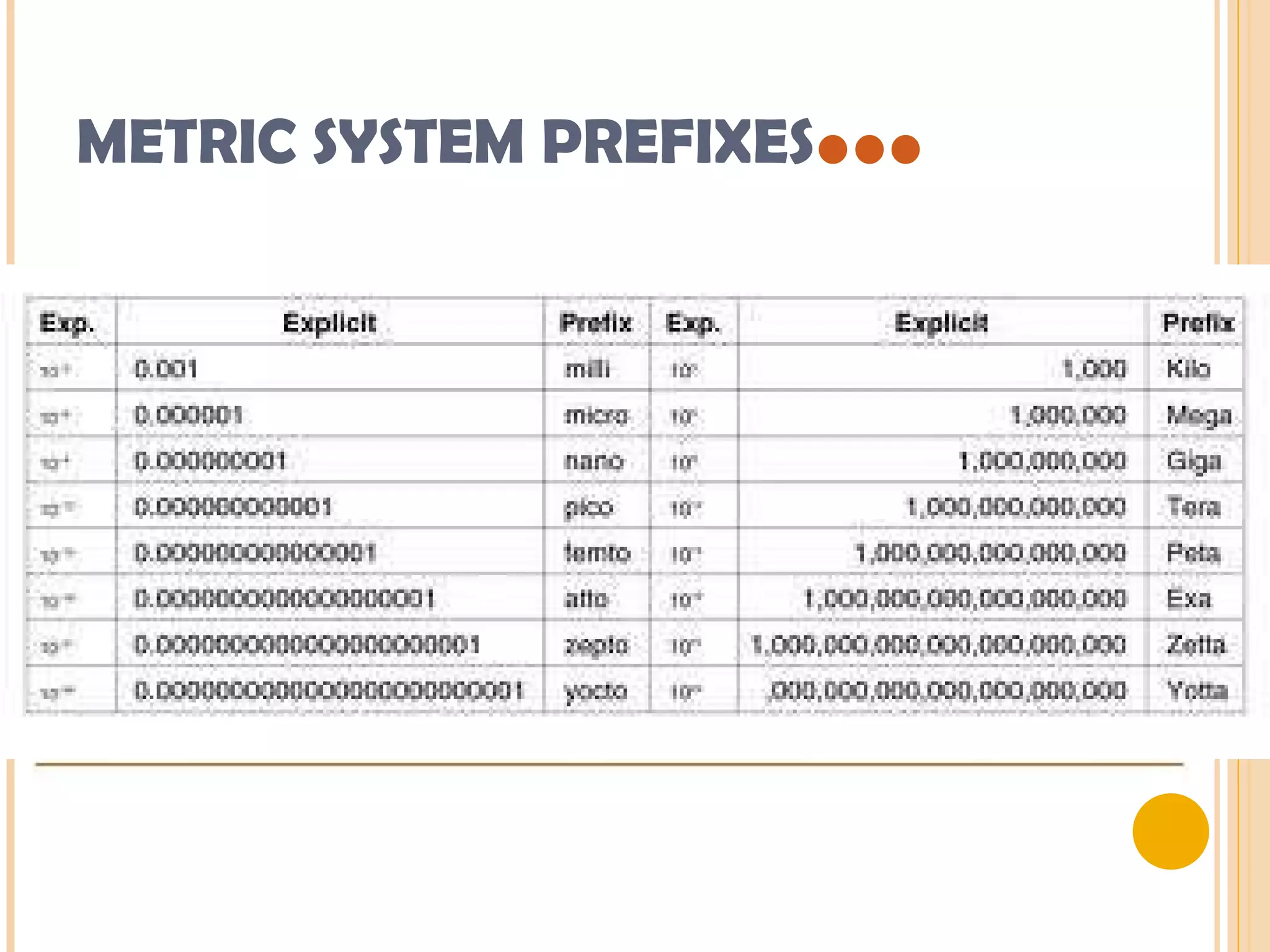
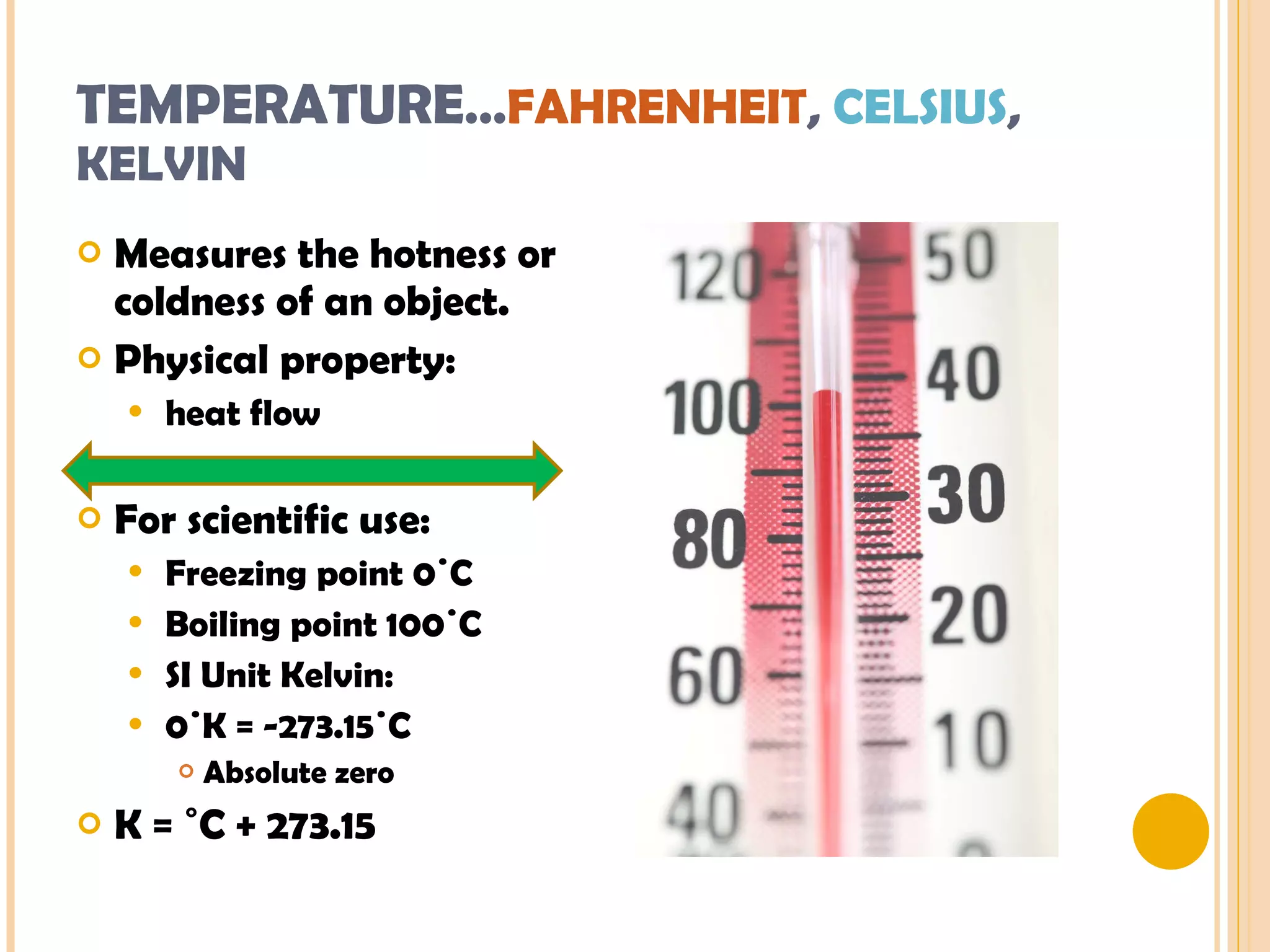
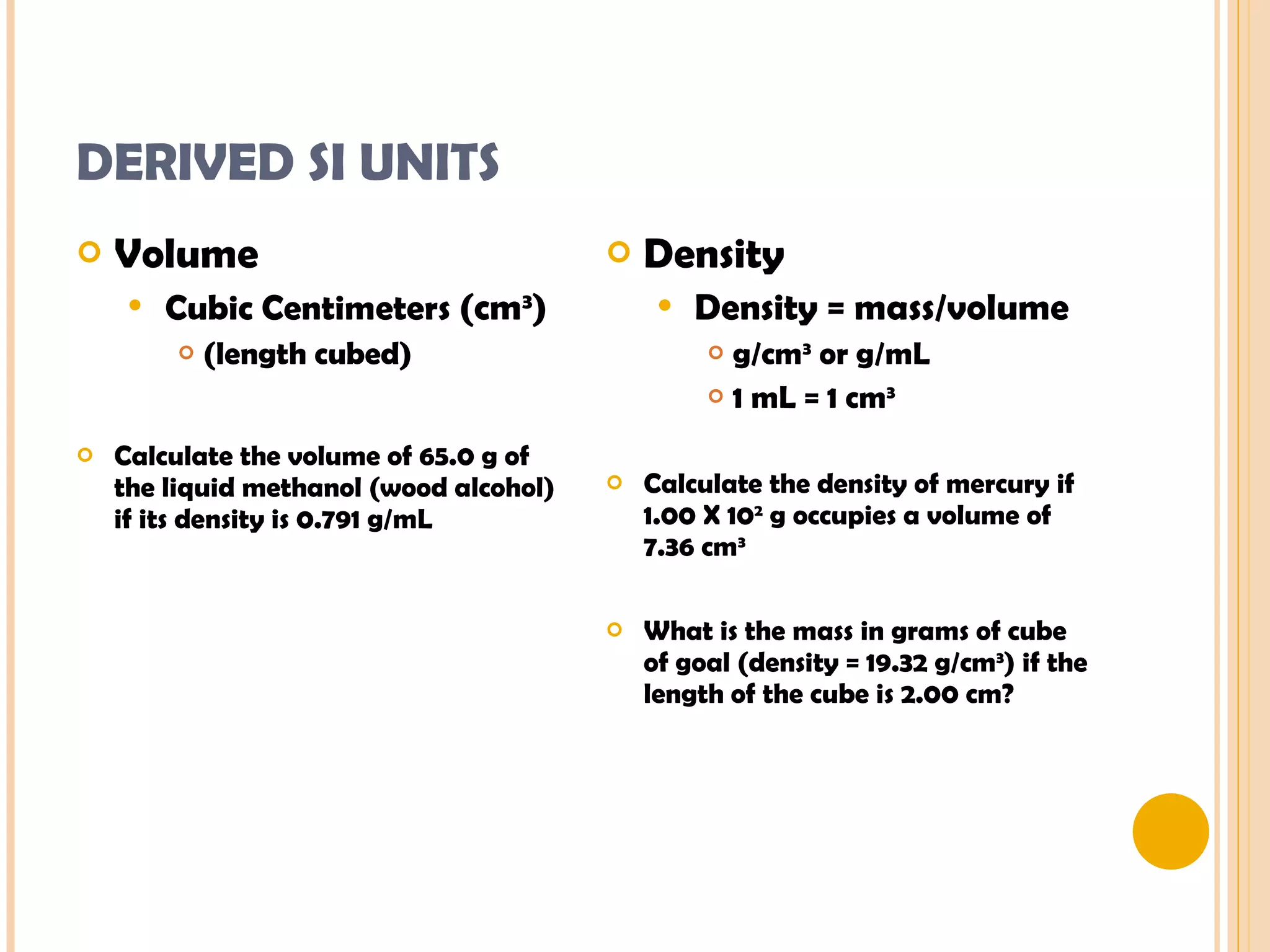
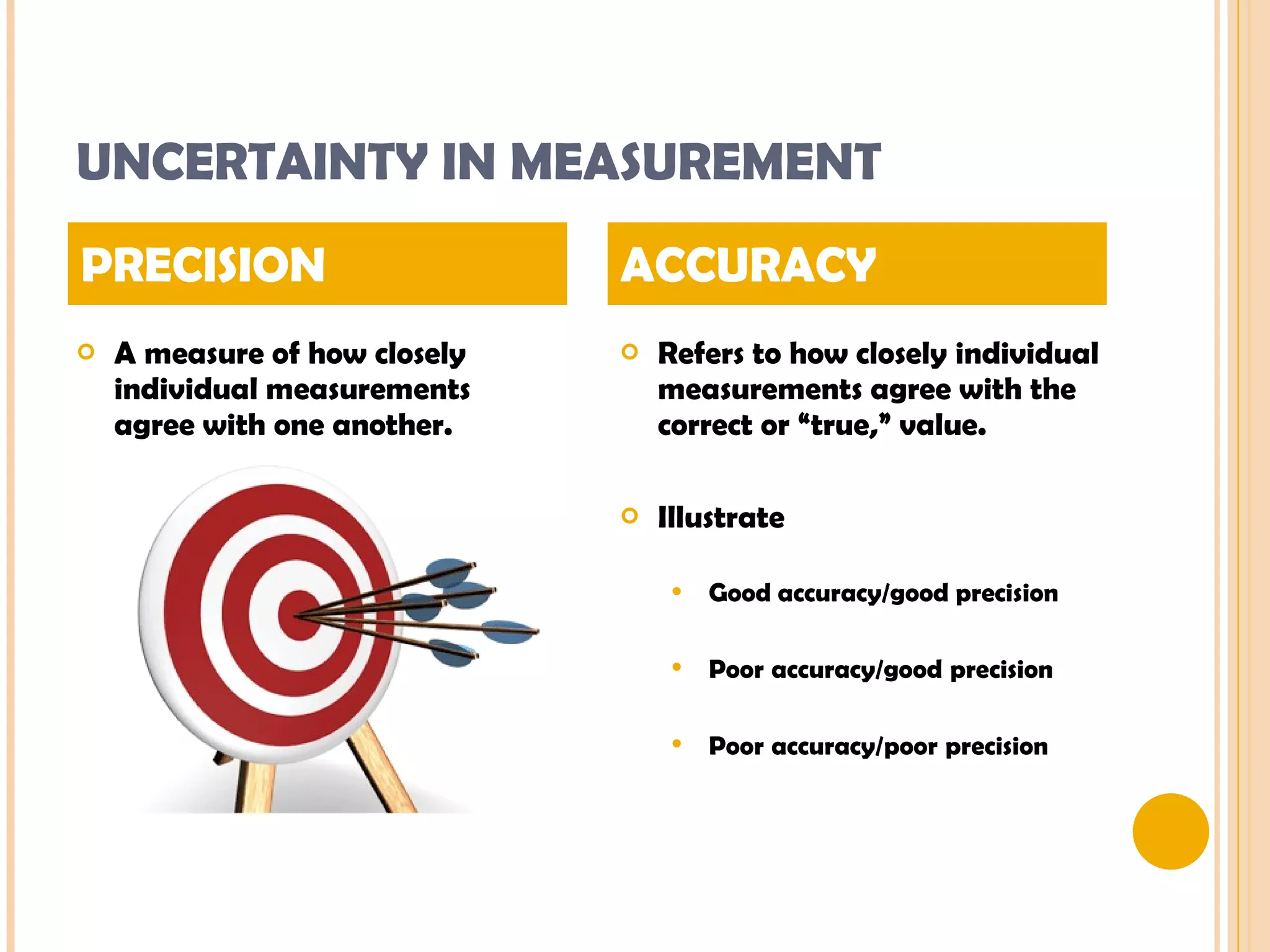
This document discusses the fundamental concepts of chemistry including the common origin of all matter from basic atomic structure. It explains that all elements are made up of different combinations of only about 100 basic elements which combine to form molecules. The document then covers key chemistry concepts like the states of matter, properties of matter, measurement units, and significant figures which are essential for understanding chemistry.