Embed presentation



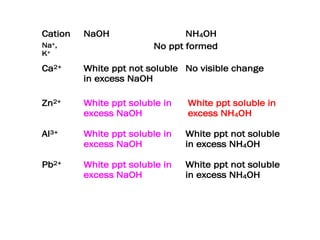

This document discusses reagents used to test for cations and identifies several common cations that can be tested for. Sodium hydroxide and ammonia solutions are used as they contain hydroxide ions, which will react with most metal ions to form precipitates of insoluble metal hydroxides. The cation can then be identified based on the color and solubility of the precipitate, with the document listing sodium, potassium, calcium, aluminium, lead, zinc, iron(II), iron(III), copper(II) and ammonium as some common cations that can be identified.




