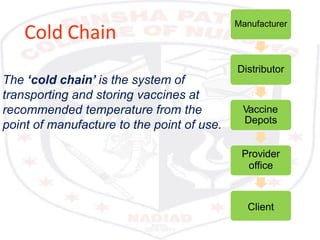
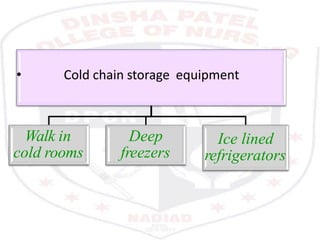
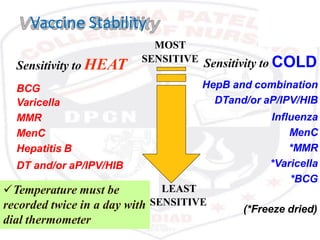
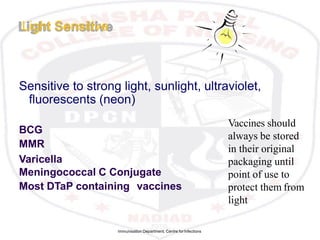
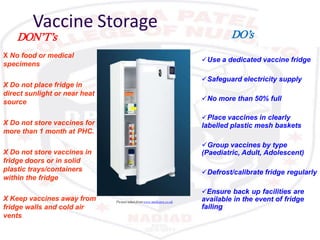
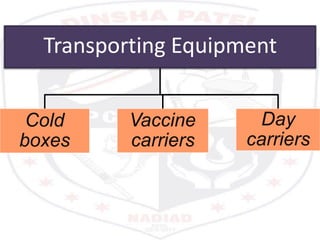
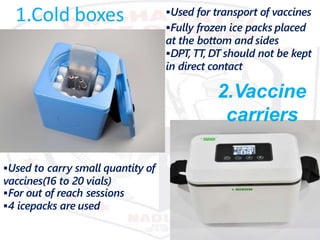
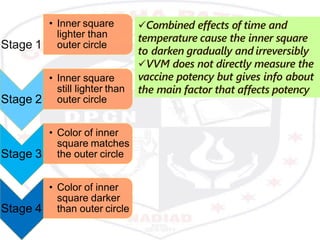
This document discusses the importance of maintaining a cold chain for vaccine storage and transportation. It outlines how vaccines must be kept within specific temperature ranges to maintain potency from the manufacturer to the point of use. Several types of cold storage equipment are described, including walk-in cold rooms, deep freezers, and ice-lined refrigerators. Proper vaccine handling and storage is essential to ensure vaccines are effective and public health programs maintain public confidence.