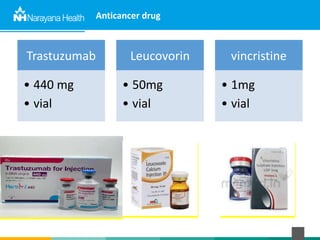
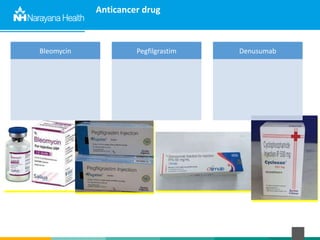
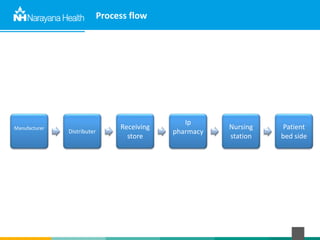
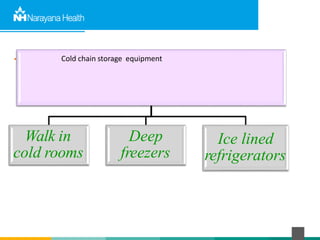
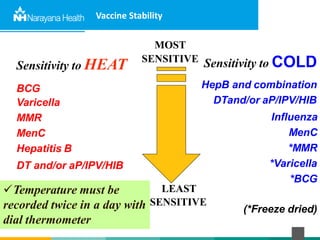
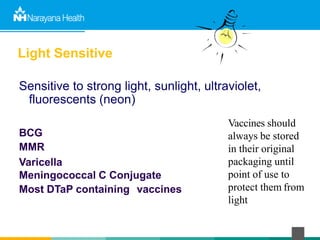
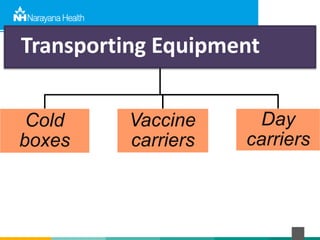
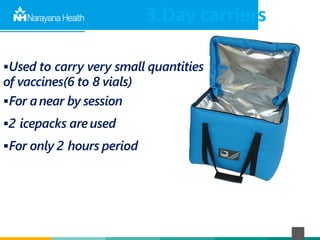
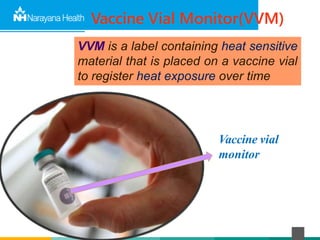
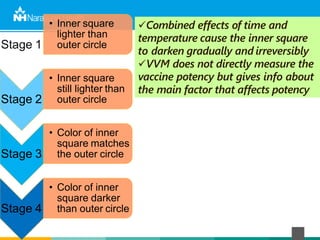
The document discusses the cold chain process for transporting and storing anticancer drugs and vaccines. It explains that vaccines and drugs must be kept within strict temperature ranges to maintain potency. The cold chain involves storage and transportation between manufacturers, distributors, receiving stores, pharmacies, and patient bedsides using equipment like cold rooms, freezers, and refrigerators. Vaccines have varying sensitivity to heat and cold and some are also light sensitive. Proper storage, transport, temperature monitoring, and backup plans are necessary to ensure drugs and vaccines do not lose potency and remain effective. Vaccine vial monitors provide a visual indicator of cumulative heat exposure over time.