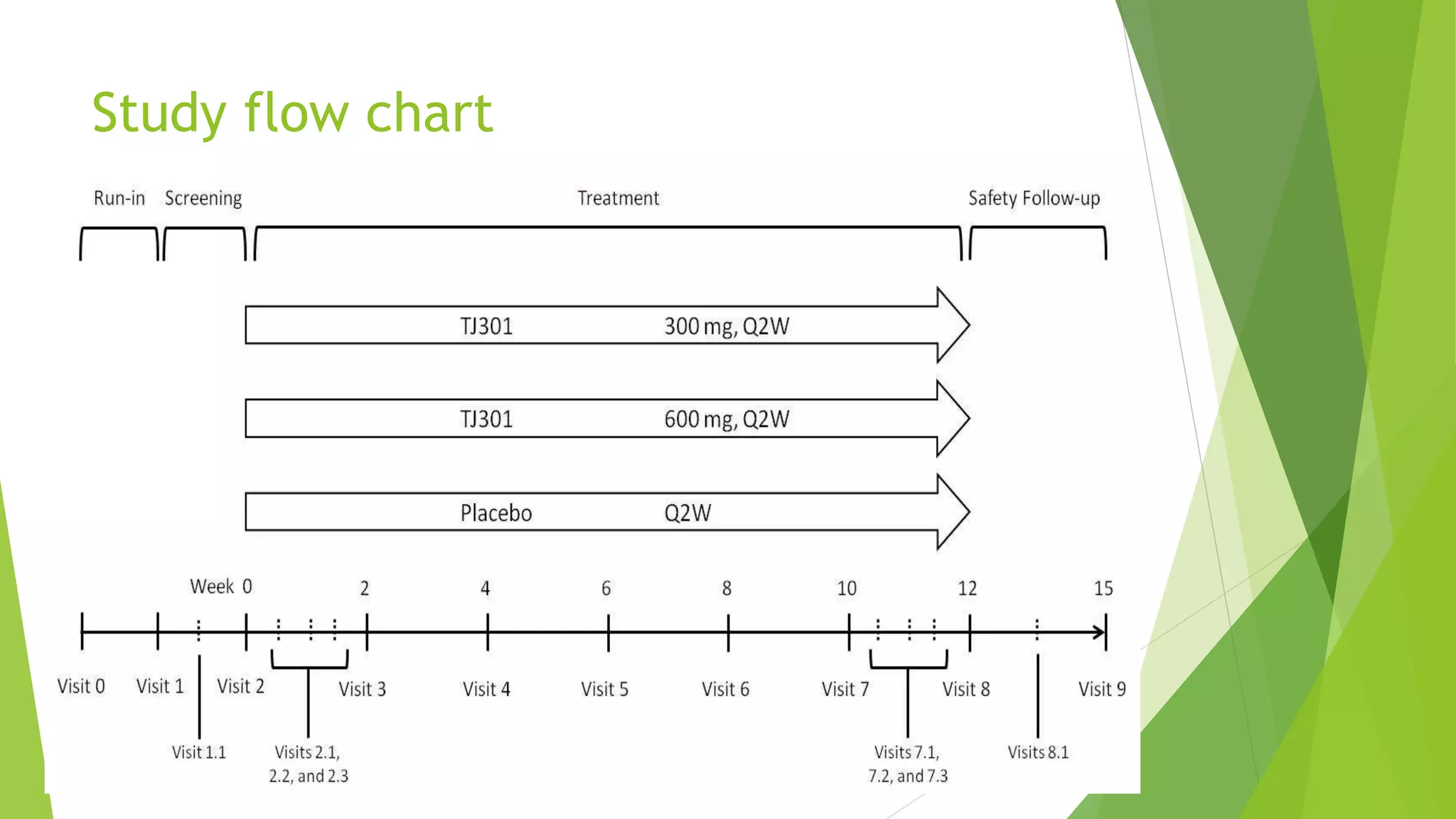
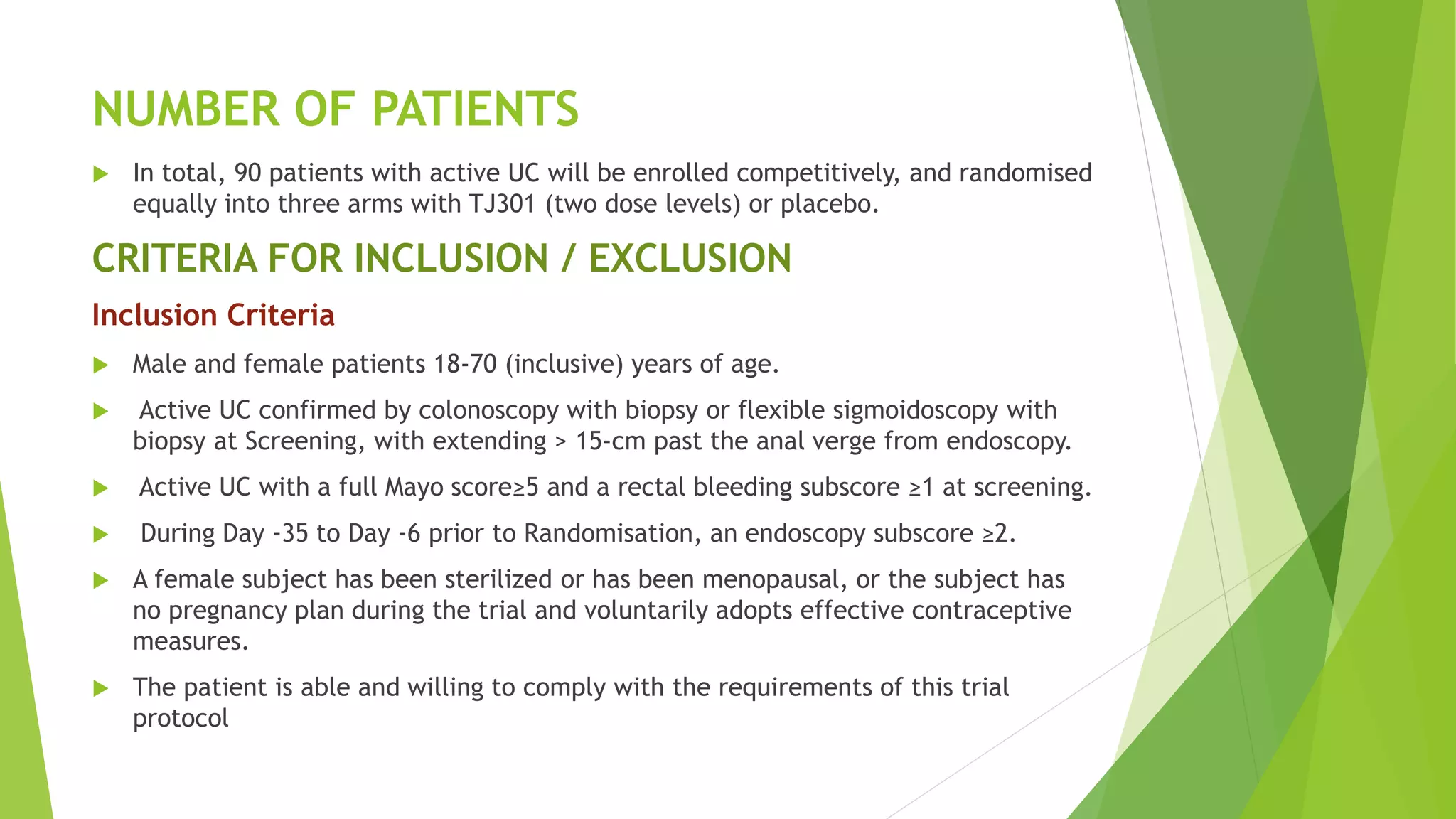
This clinical trial protocol summarizes a phase 2 double-blind randomized placebo-controlled trial to evaluate the safety and efficacy of TJ301 for the treatment of active ulcerative colitis. The trial will enroll 90 patients to receive either 600mg of TJ301 biweekly, 300mg of TJ301 biweekly, or placebo biweekly for 12 weeks. The primary endpoint is clinical and endoscopic remission at week 12. Secondary endpoints include safety assessments, pharmacokinetic measures, and changes in disease activity scores from baseline to week 12. The protocol outlines the study design, patient selection criteria, treatments, assessments, data management, and statistical analysis plan.