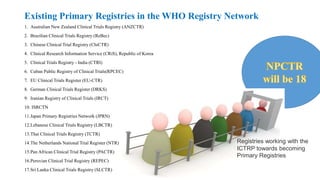
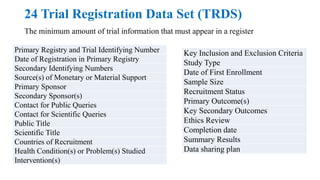
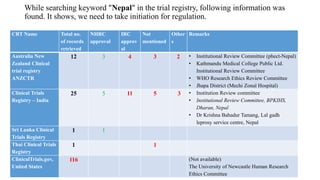
The document discusses the importance of clinical trial registration in Nepal, emphasizing compliance with international standards and guidelines for transparency, prevention of duplicative research, and facilitating patient recruitment. It outlines the structure and requirements of the Nepal Clinical Trial Registry (NPCTR) in accordance with the World Health Organization's International Clinical Trials Registry Platform (ICTRP). The NPCTR aims to support safe and ethical clinical trials in Nepal through rigorous auditing and adherence to regulatory frameworks.