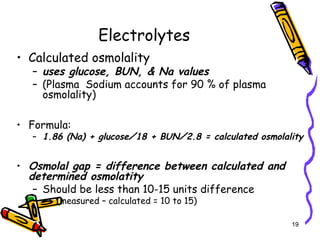
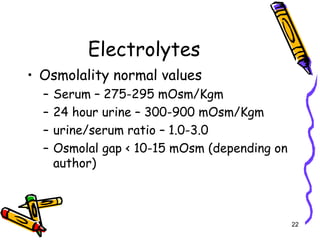
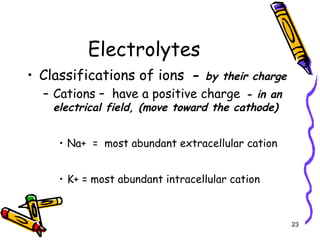
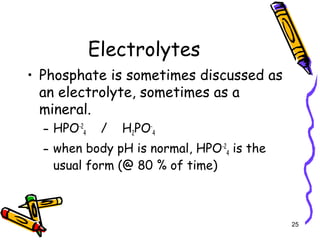
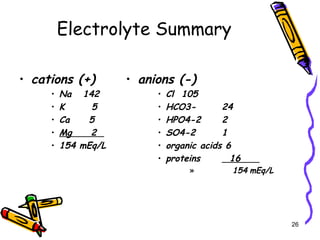
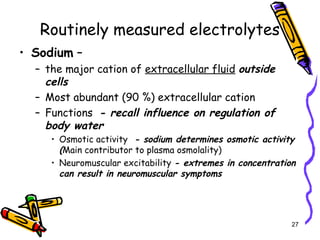
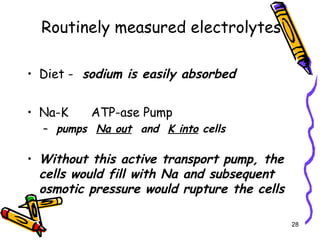
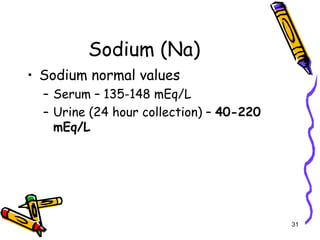
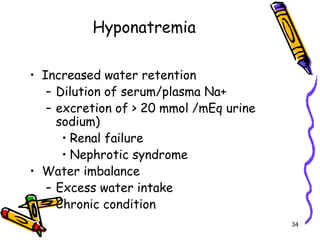
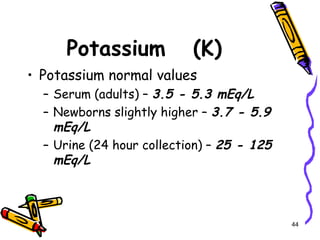
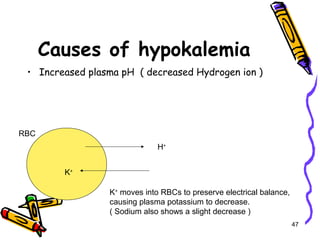
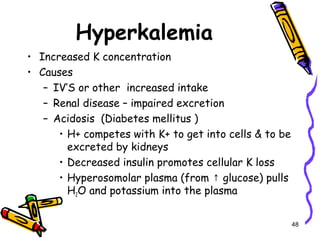
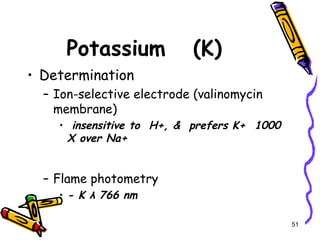
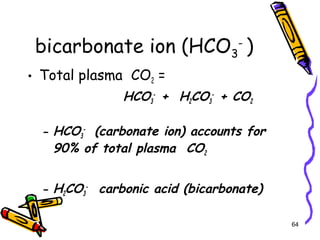
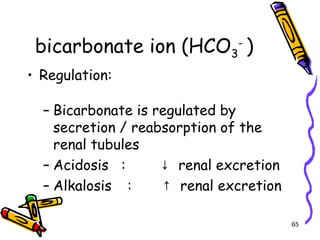
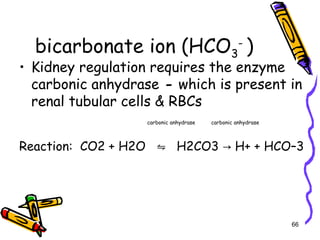
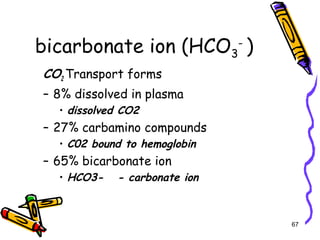
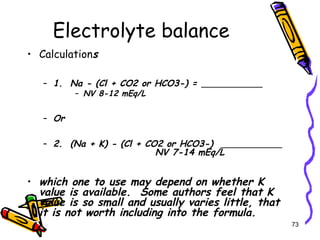
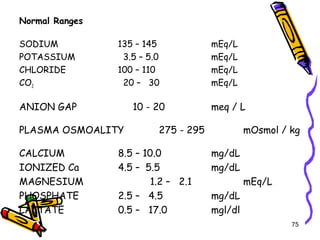
The document covers the essentials of clinical chemistry related to electrolytes, including their definitions, physiological functions, and significance in body hydration regulation. Key electrolytes such as sodium, potassium, and chloride are highlighted, alongside their roles in osmotic pressure, neuromuscular excitability, and acid-base balance. Laboratory assessments of hydration status and methods for determining electrolyte concentrations are also discussed.