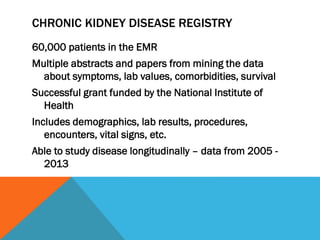
Clinical research informatics involves using informatics tools to support clinical research activities like managing clinical trials data and conducting secondary research using clinical data. It can help make clinical trials more efficient by facilitating study design, subject recruitment from electronic medical records and social media, data collection and management, and data analysis. National initiatives aim to develop informatics tools and networks to help researchers access data and accelerate clinical research.