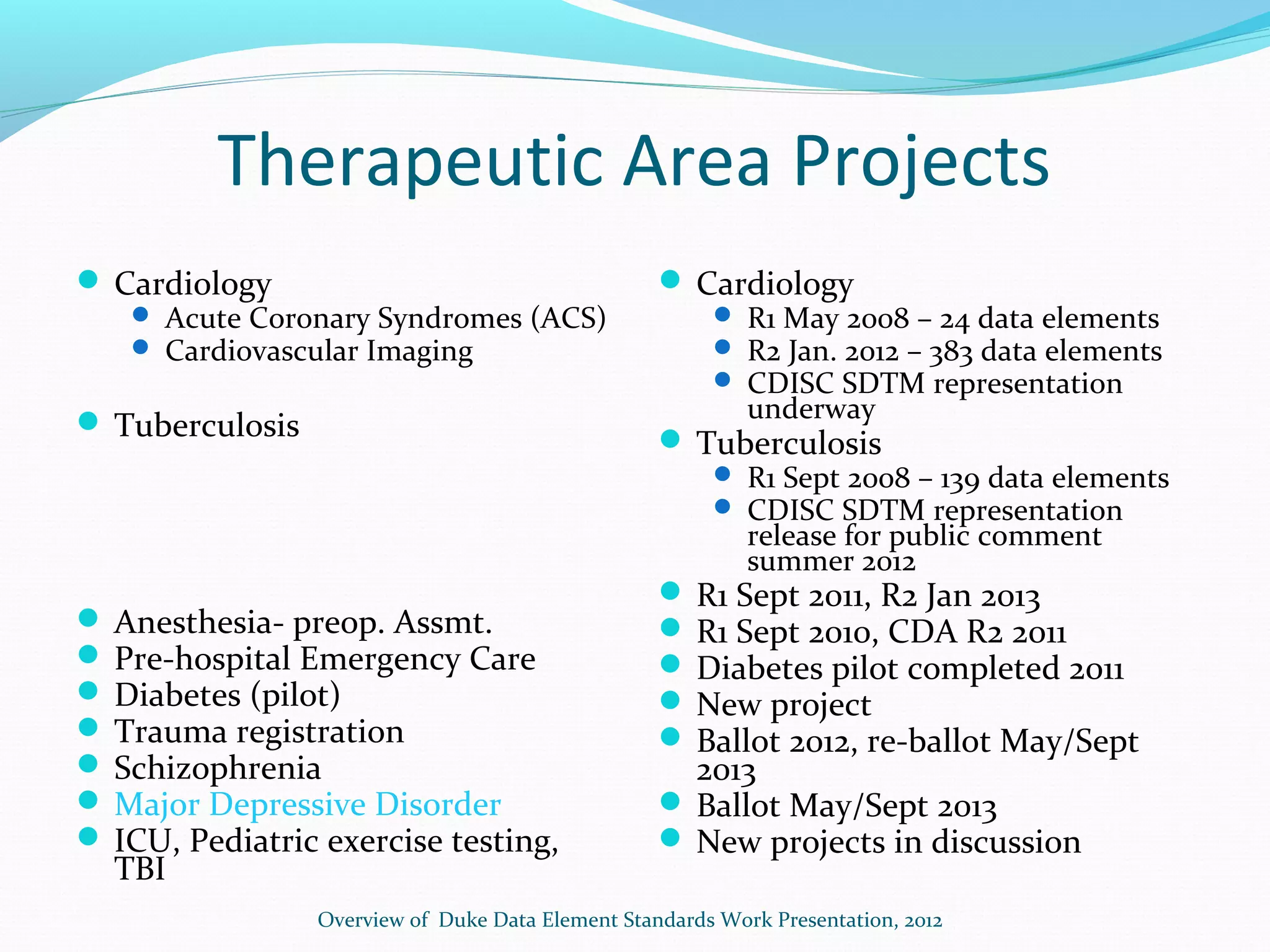
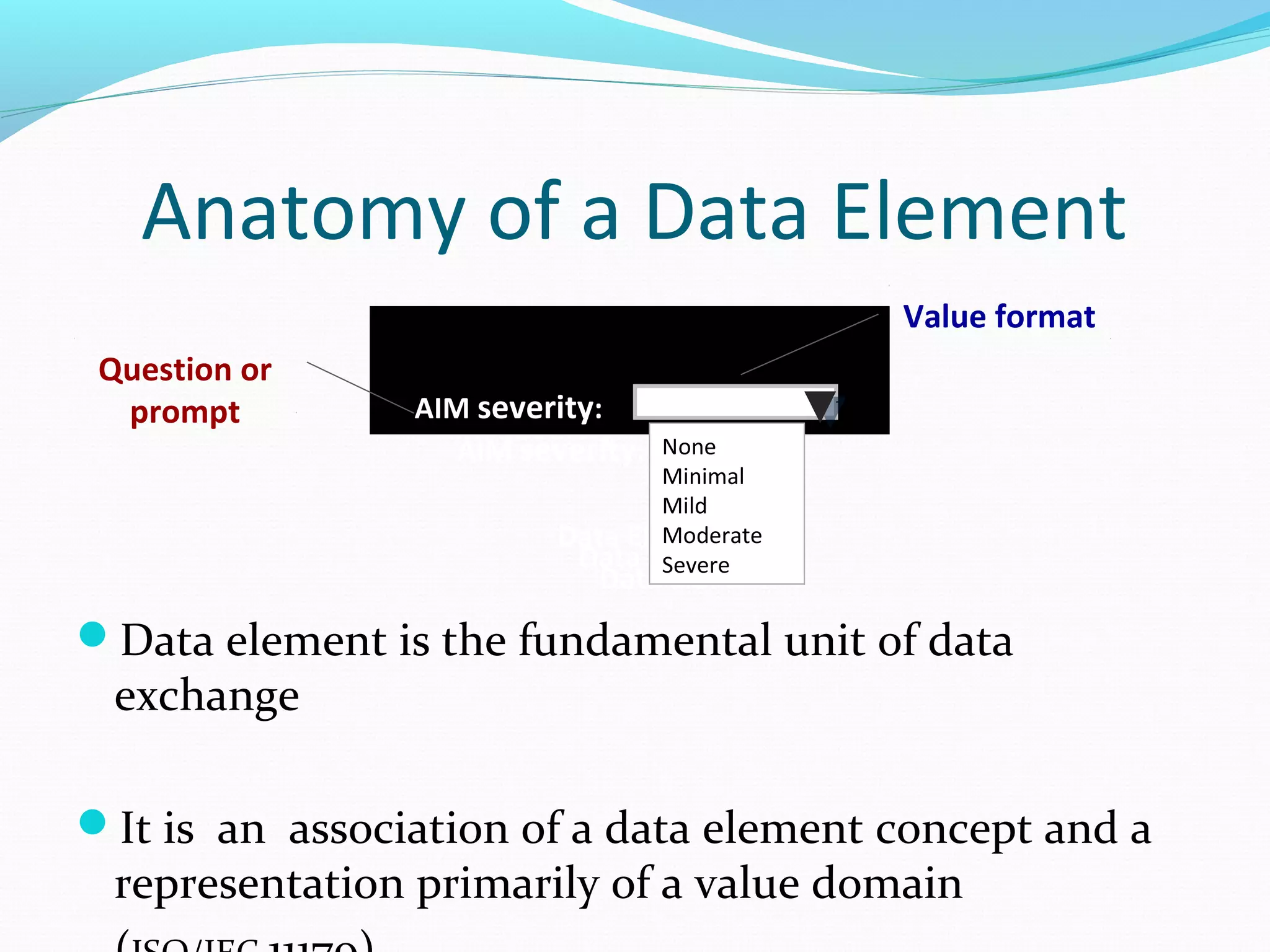
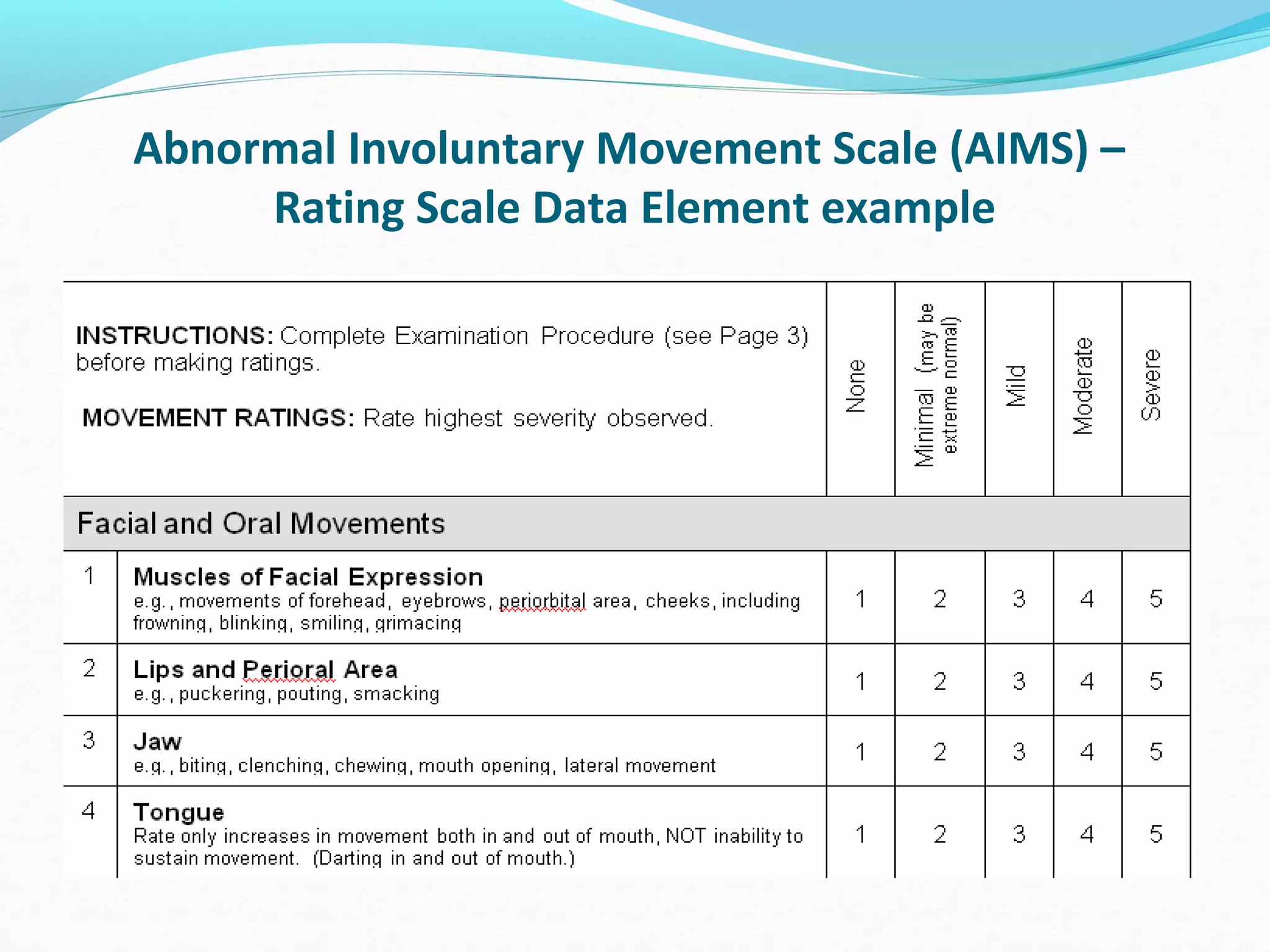
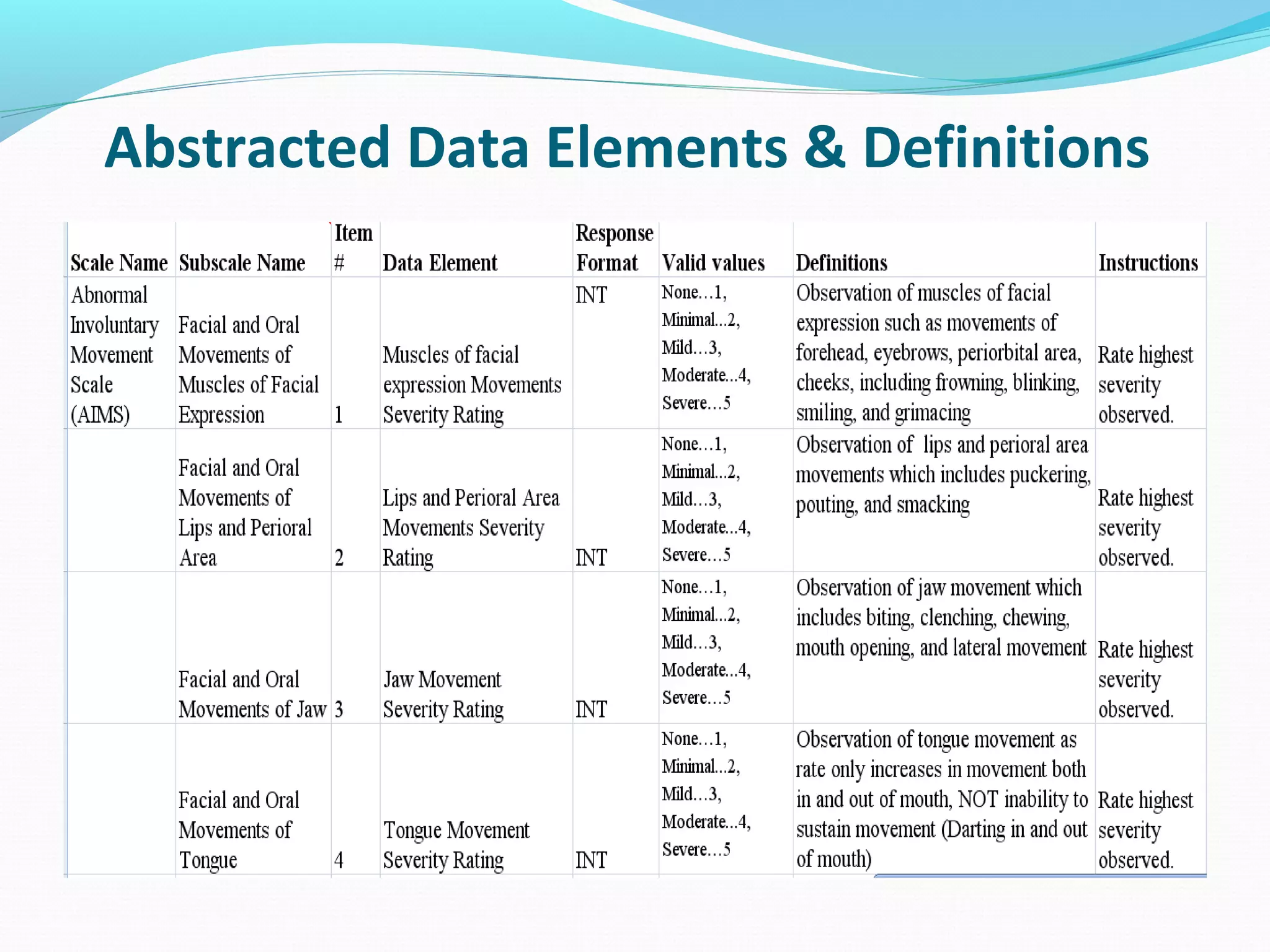
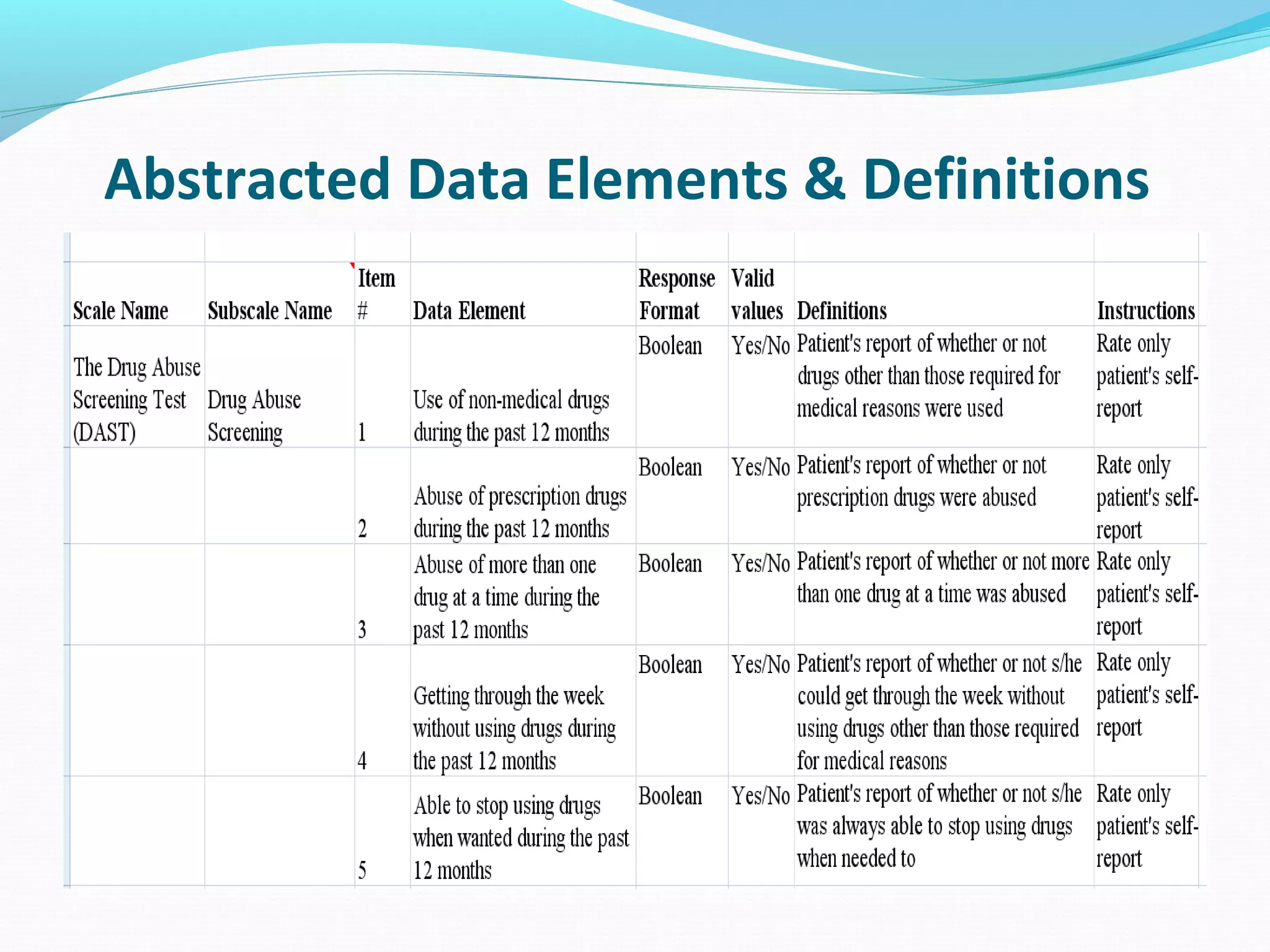
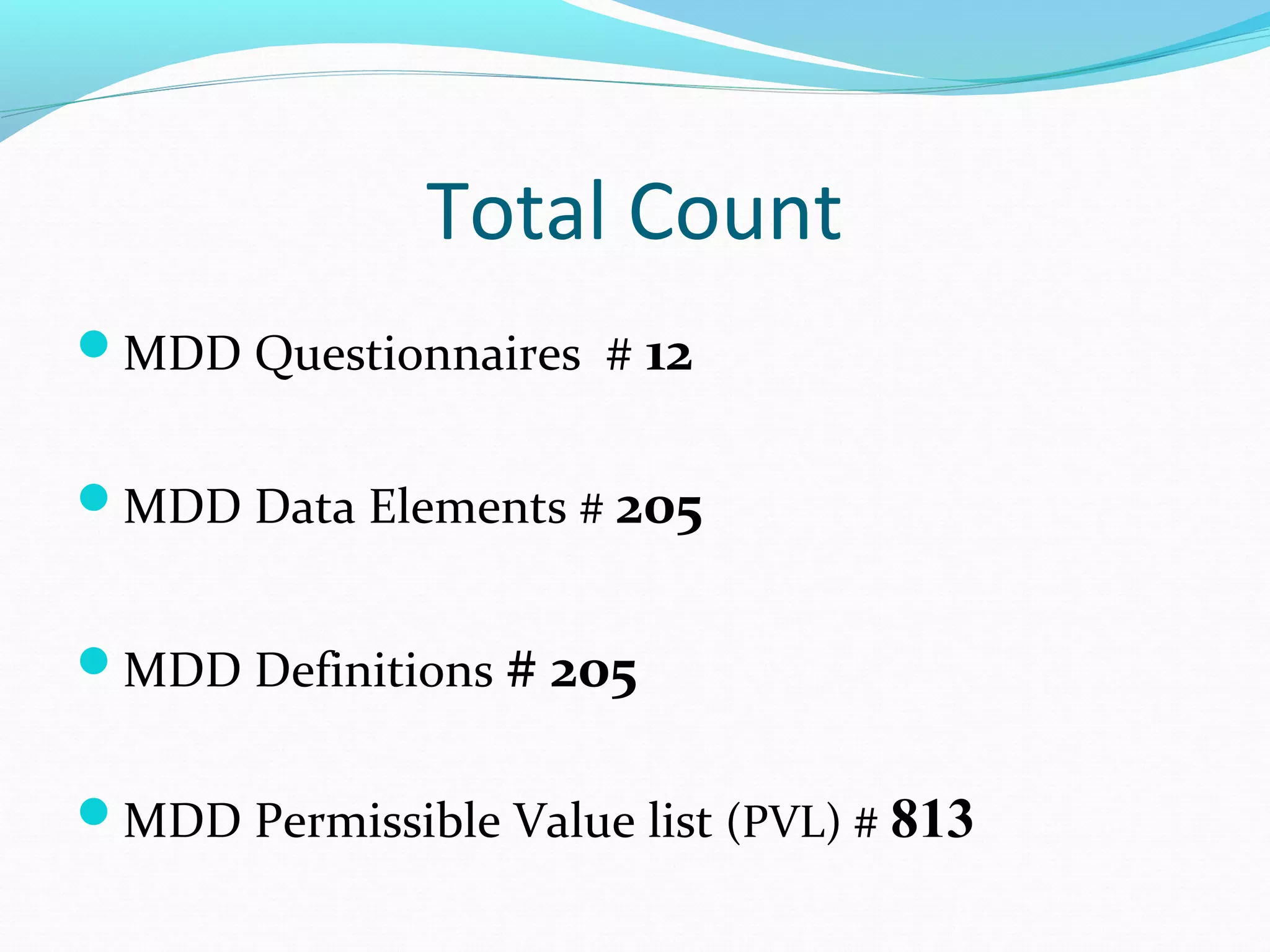
This document discusses problems with current clinical data standards and proposes standardizing data elements as a solution. It notes that while terminologies are useful, variations in meaning and coding make exchanging and reusing clinical data difficult. Standardizing common data elements could help address this by facilitating semantic interoperability and improving data quality for patient care and secondary uses. The document outlines efforts by the Clinical Data Interchange Standards Consortium (CDISC) and Health Level 7 (HL7) to develop standardized data element definitions and value sets for various clinical domains through a multi-stakeholder process.