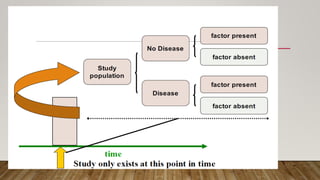
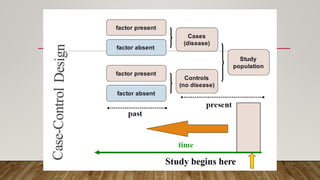
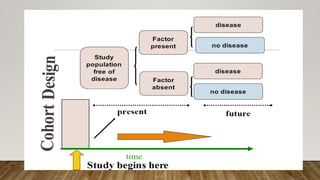
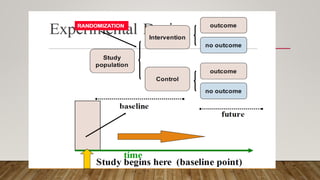
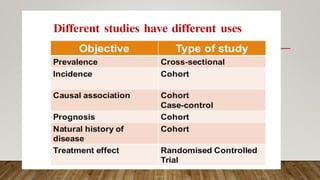
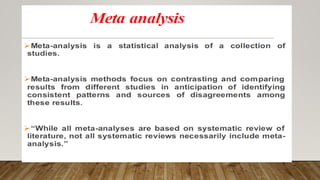
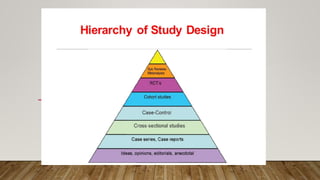
This document discusses medical research study designs and biostatistics. It defines biostatistics as the application of statistics to biological sciences, medicine, and public health. Biostatistics is key to conducting clinical trials and is important for evidence-based medicine. Various types of studies are described including observational studies like descriptive, ecological, cross-sectional, case-control and cohort studies. Experimental studies include randomized controlled trials. Randomized controlled trials are described as one of the most powerful study designs where participants are randomly allocated to treatment or control groups to reduce bias.