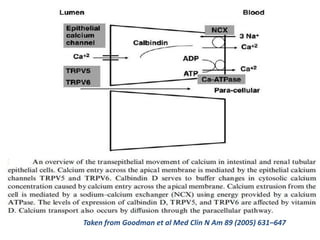
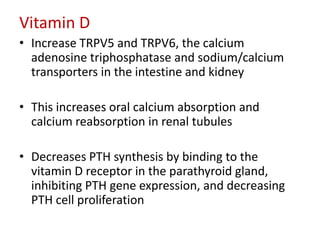
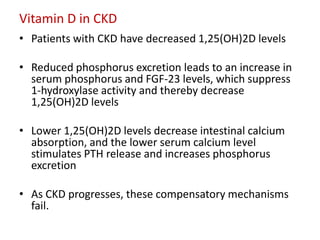
CKD-MBD is a systemic disorder seen in progressive kidney disease characterized by abnormalities in calcium, phosphorus, PTH, and vitamin D levels as well as bone abnormalities and soft tissue calcification. Key aspects of CKD-MBD include impaired regulation of phosphorus and calcium leading to elevated levels that stimulate PTH production and reduced vitamin D activation. This disrupts bone and mineral homeostasis and increases cardiovascular risks. Treatment involves controlling levels through diet, phosphate binders, vitamin D, and PTH therapies according to KDIGO guidelines.



![Definition of CKD-MBD
A systemic disorder of mineral and bone
metabolism due to CKD manifested by either one
or a combination of the following:
• Abnormalities of calcium, phosphorus, PTH, or
vitamin D metabolism
• Abnormalities in bone turnover, mineralization,
volume, linear growth, or strength
• Vascular or other soft-tissue calcification
Reference: KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation,
Prevention, and Treatment of CKD-MBD(Kidney Int. 2009; 76[suppl 113])](https://image.slidesharecdn.com/ckd-mbd-120722054927-phpapp02/85/Chronic-Kidney-Disease-MBD-Part-1-4-320.jpg)


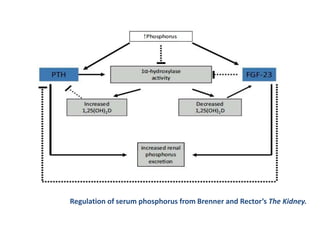
![FGF-23 has the following actions
• Downregulates luminal sodium/phosphate cotransporters in the
proximal tubule, decreasing phosphorus reabsorption and therefore
increasing its excretion
• Inhibits 1-hydroxylase, decreasing the conversion of 25-
hydroxyvitamin D (25[OH]D) to 1,25-dihydoxyvitamin D
(1,25[OH]2D3; calcitriol
• Stimulates 24-hydroxylase (CYP24), leading to vitamin D
degradation
• Inhibits PTH secretion](https://image.slidesharecdn.com/ckd-mbd-120722054927-phpapp02/85/Chronic-Kidney-Disease-MBD-Part-1-9-320.jpg)