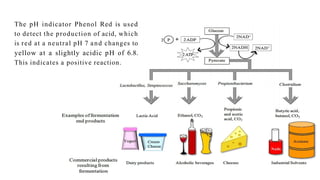
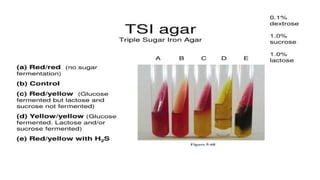
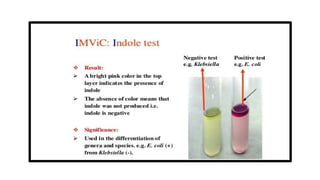
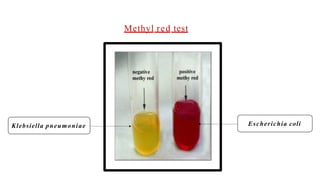
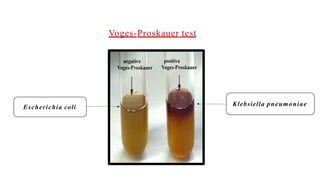
The document outlines biochemical activities and tests for the identification of bacteria, emphasizing that unique biochemical reactions act as a 'thumbprint' for each species due to their distinct DNA and enzyme production. It details various tests including carbohydrate fermentation, triple sugar iron agar, urease, oxidase, nitrate, catalase, coagulase, gelatinase, and the IMViC tests, each assessing different biochemical capabilities. These tests are crucial in differentiating among species, particularly within the Enterobacteriaceae family and other relevant bacteria.