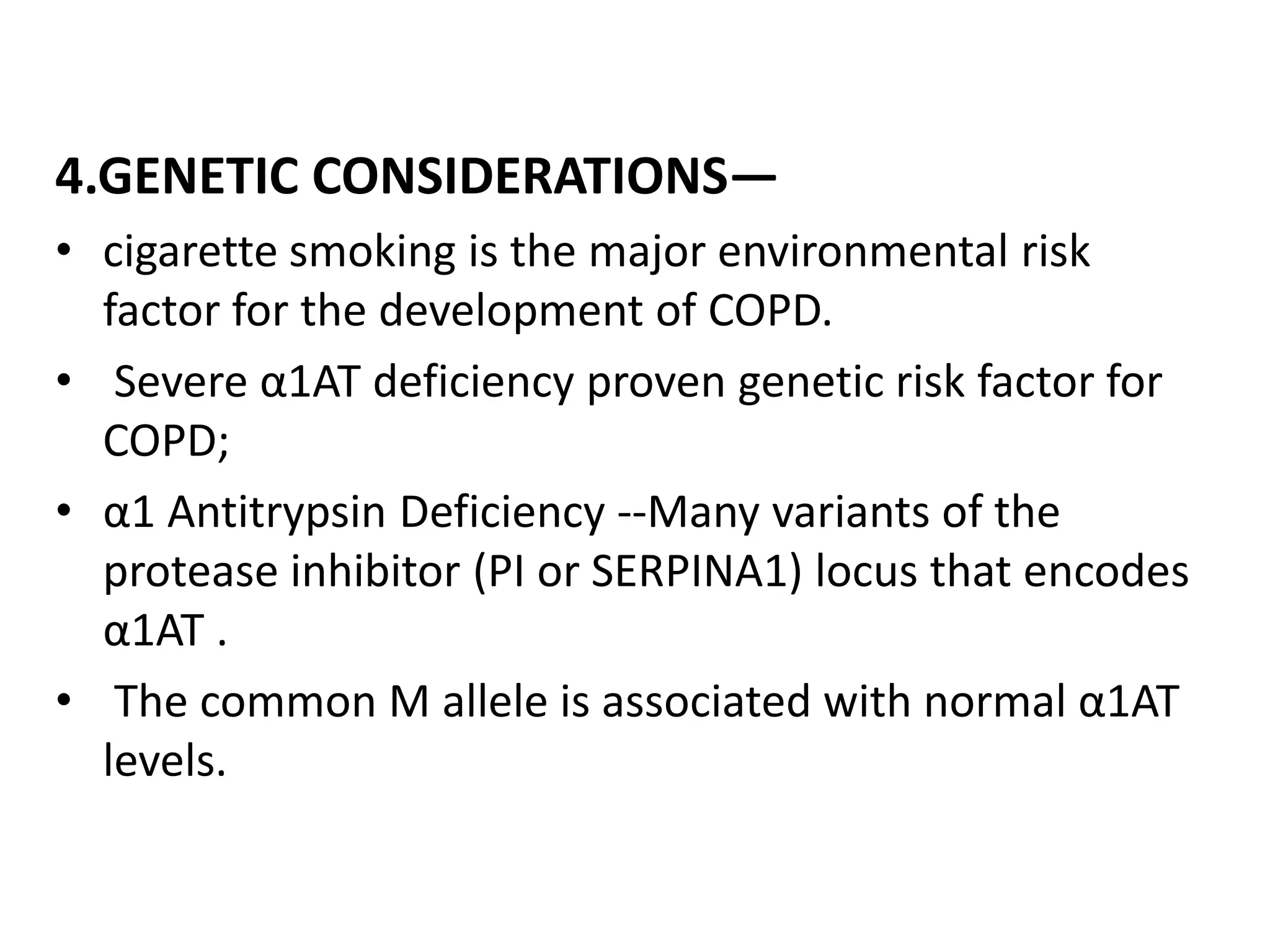
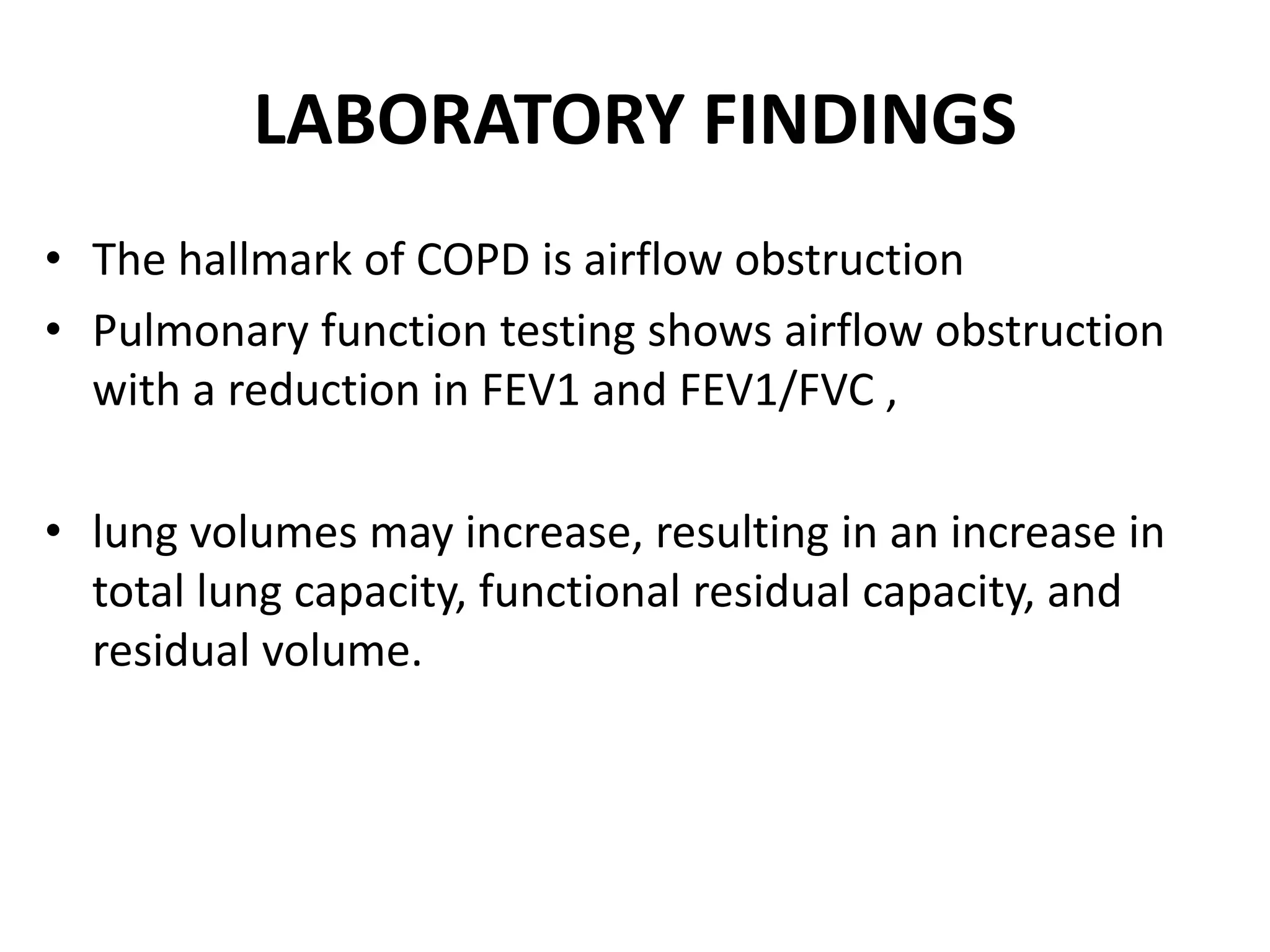
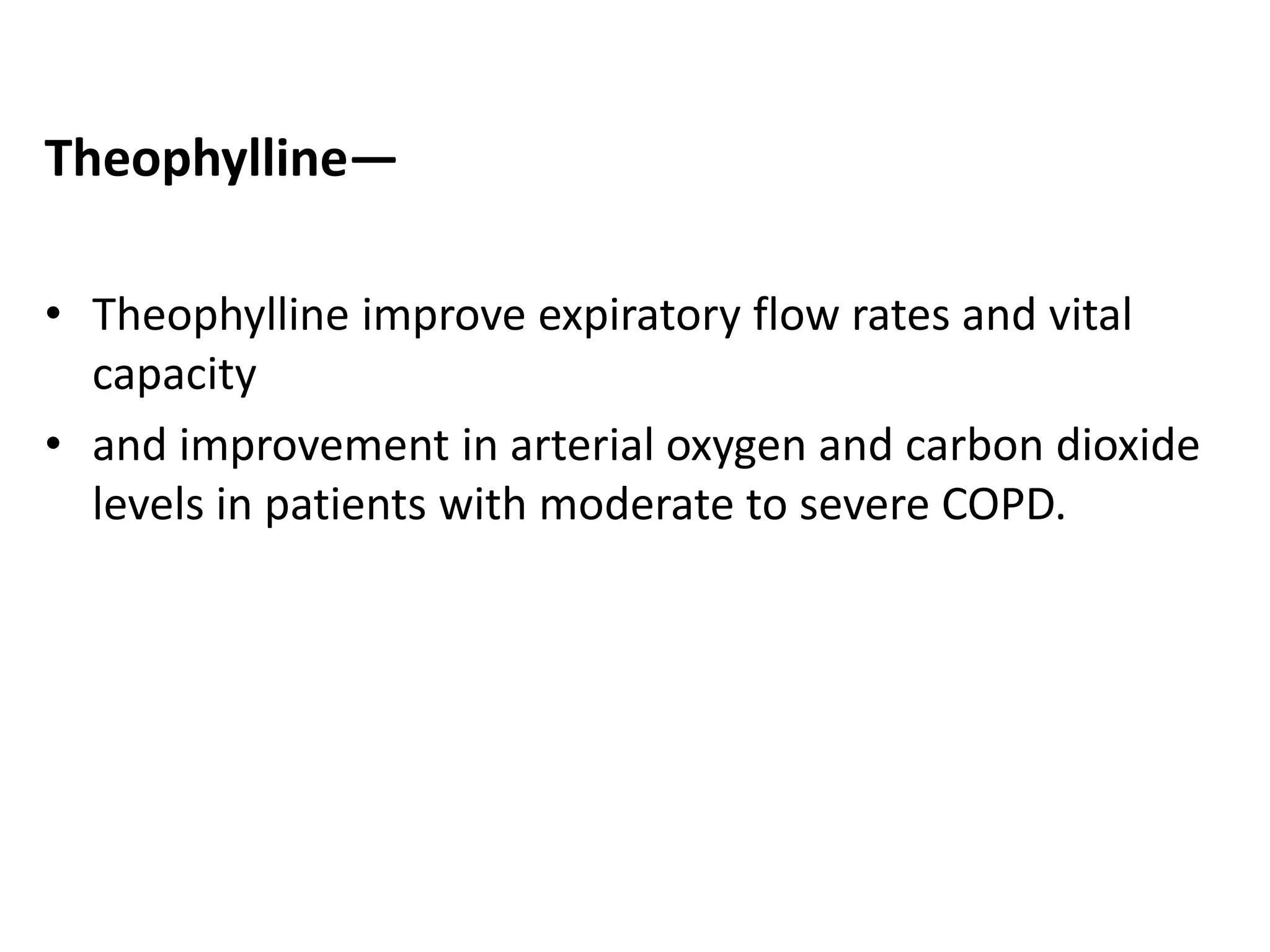
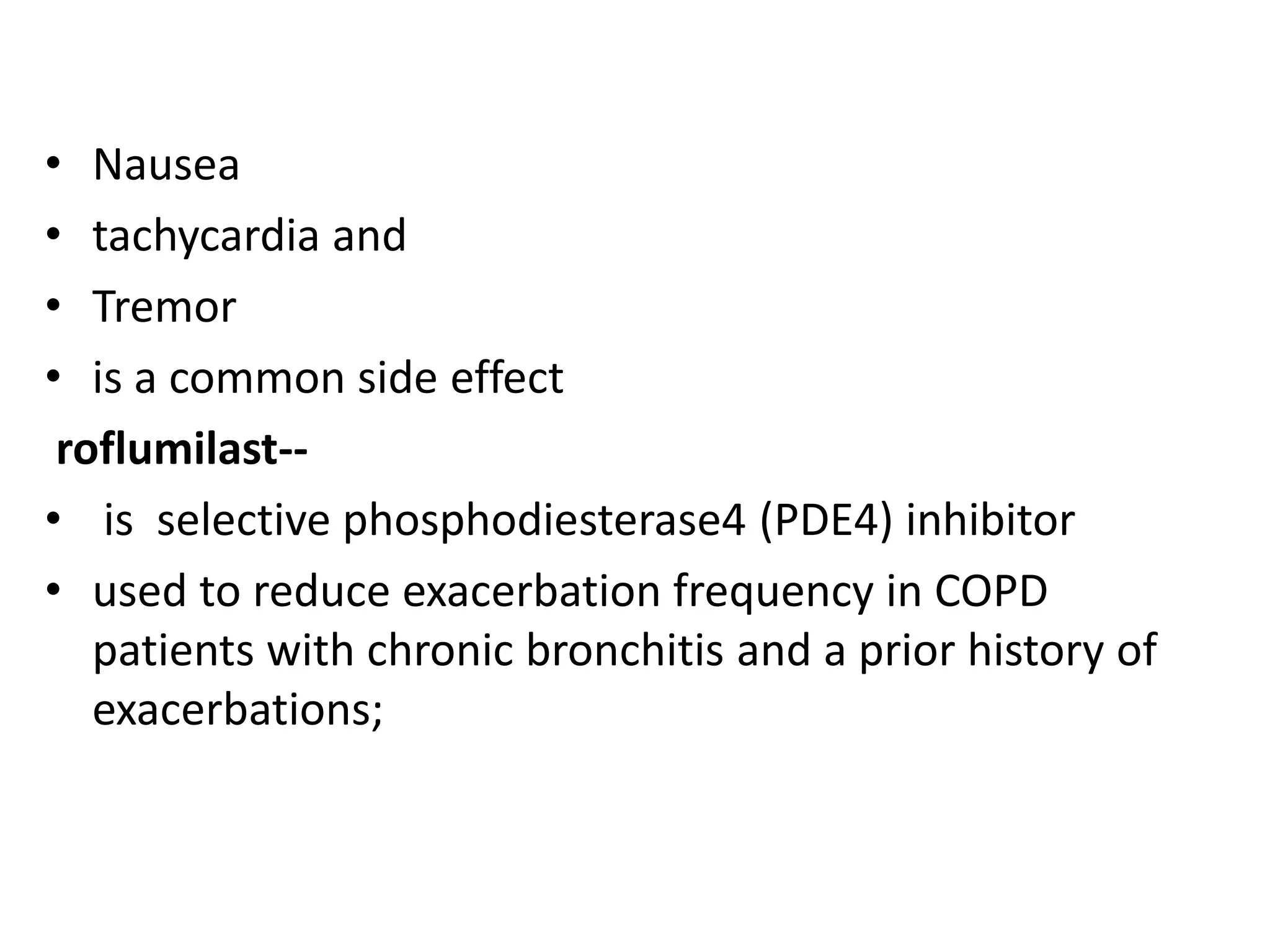
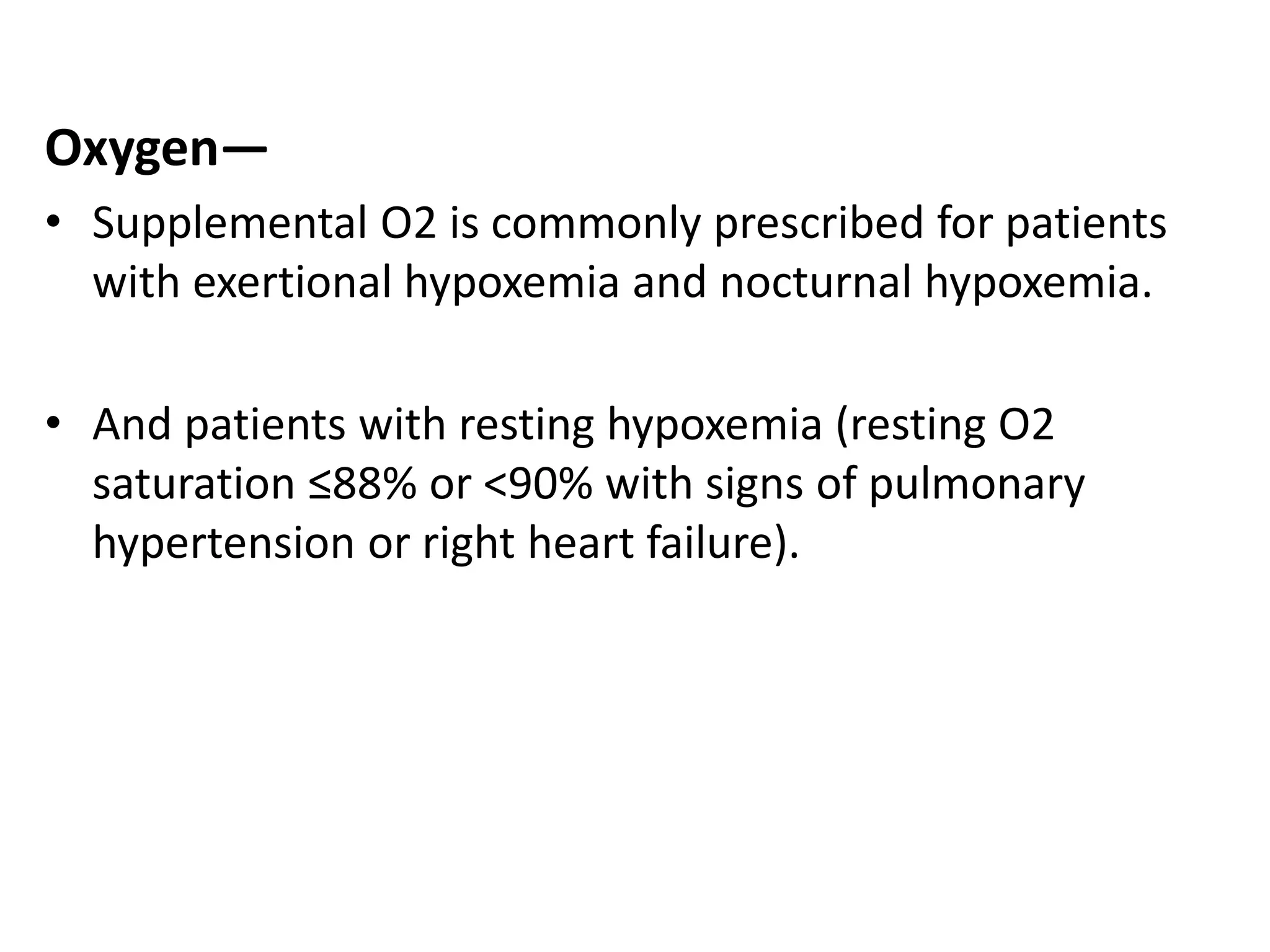
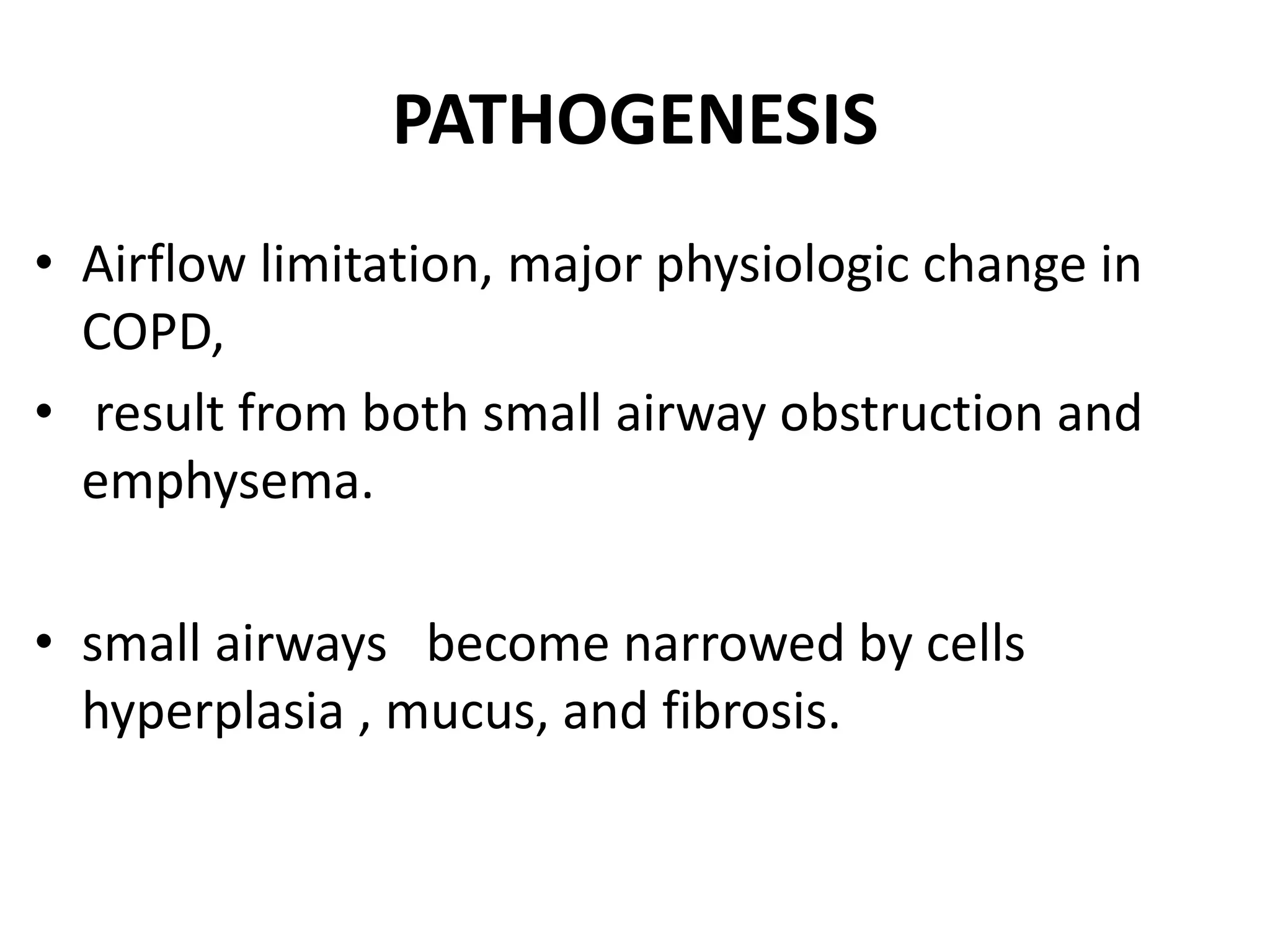
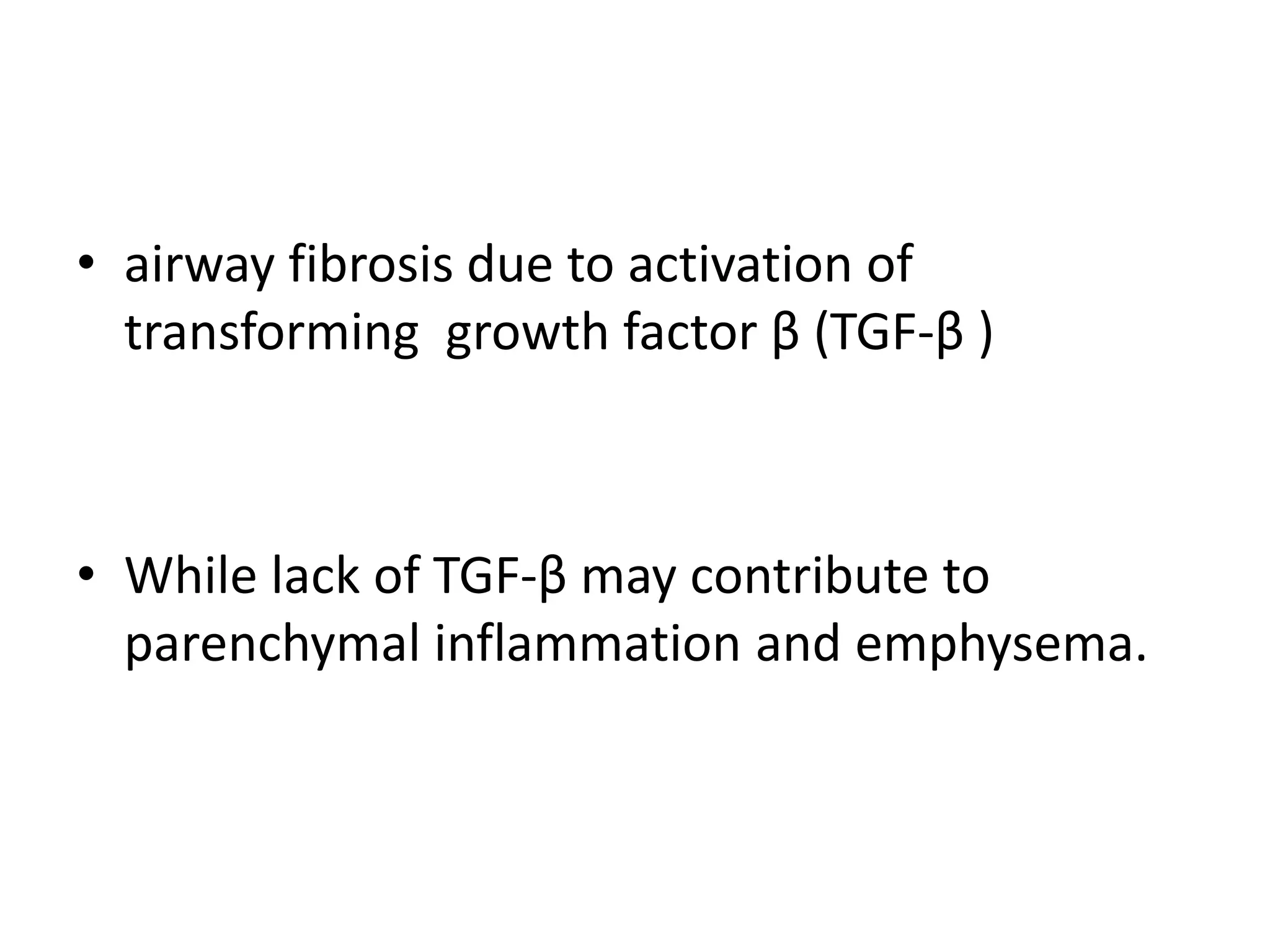
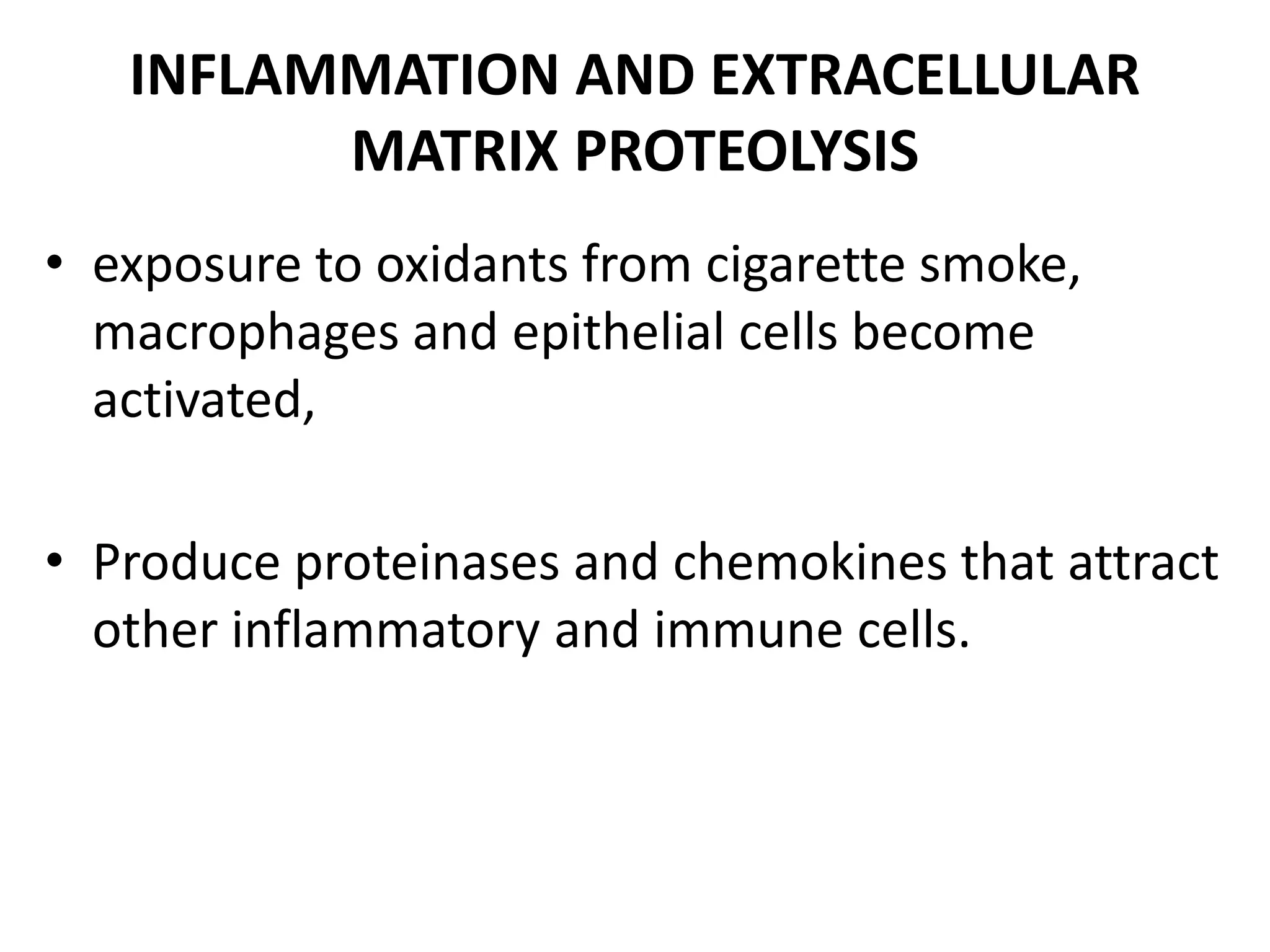
This document discusses chronic obstructive pulmonary disease (COPD). It defines COPD as a chronic, progressive lung disorder characterized by limited airflow. The document covers the pathogenesis of COPD, which involves inflammation and damage to lung tissue from cigarette smoke exposure. This leads to emphysema and obstruction of small airways. The clinical presentation of COPD typically includes cough, sputum production, and shortness of breath with exertion. Physical examination may reveal prolonged expiration in severe cases. The major risk factor for COPD is cigarette smoking.


























![• Key parameters obtained from spirometry
include
• the volume of air exhaled within the first second
of the forced expiratory maneuver (FEV1)
• The total volume of air exhaled during the entire
spirometric maneuver (forced vital capacity
[FVC]).](https://image.slidesharecdn.com/chronicobstructivepulmonary-180413045720/75/Chronic-obstructive-pulmonary-by-dr-shailesh-gupta-NIKHIL-A-KUMAR-32-2048.jpg)