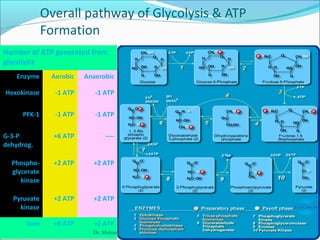
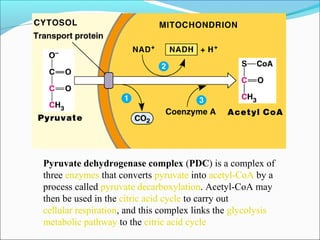
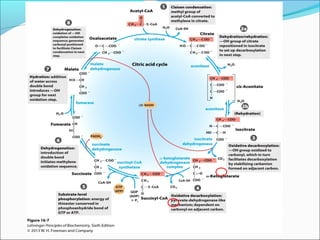
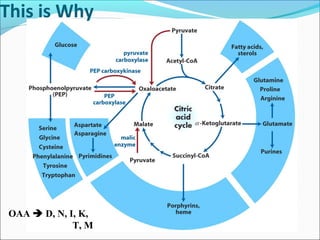
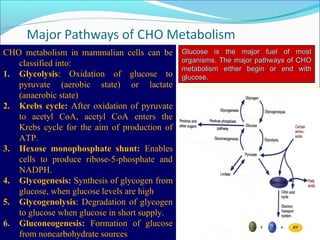
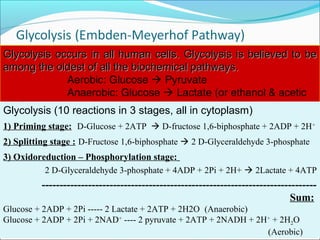
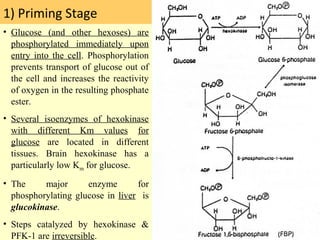
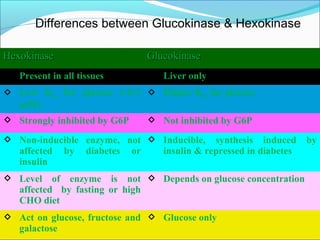
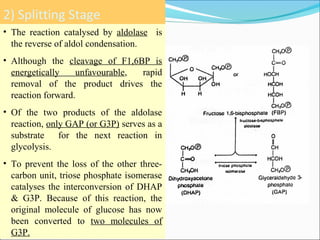
1. Glycolysis is the pathway that breaks down glucose to pyruvate, generating a small amount of ATP.
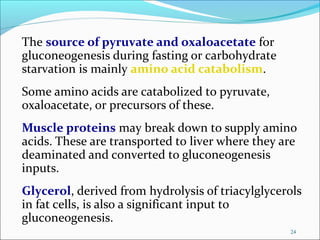
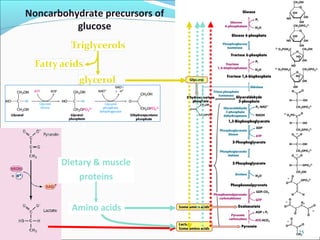
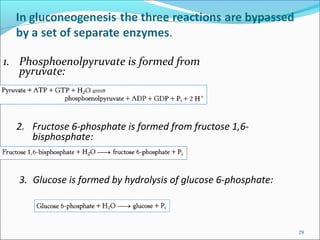
2. Gluconeogenesis is the formation of glucose from non-carbohydrate precursors like amino acids or glycerol, mainly occurring in the liver and kidneys.
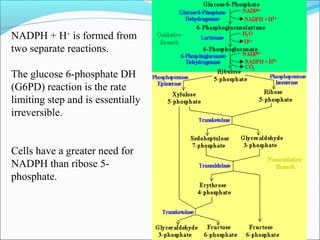
3. The hexose monophosphate shunt generates reducing power in the form of NADPH and produces ribose-5-phosphate from glucose-6-phosphate.





![March 17, 2018 Dr. Mohamed Z Gad 11
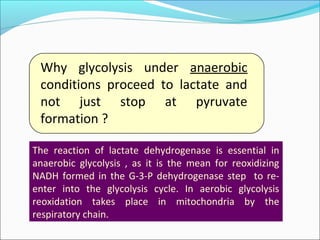
3) Oxidoreduction –
Phosphorylation
Stage• G-3-P dehydrogenase is a tetramer,
each subunit contains 1 binding site
for G3P & another for NAD+
(NAD+
is permanently bound to the enzyme).
• G3P 1,3-BPG 3PG and PEP
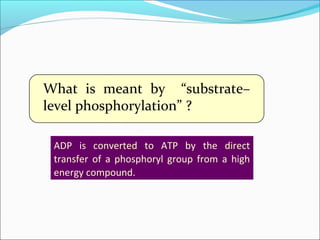
Pyruvate are examples of
“substrate–level” phosphorylation.
• 3-PG 2-PG is mediated by an
intermediate [2,3-BPG]. Most cells
have low amounts of 2,3-BPG except
in RBCs, which act as allosteric
modifier of Hb-O2 binding.
• PEP Pyruvate is an “irreversible
reaction” due to free energy loss
associated with tautomerization of
the enol to the more stable keto form.](https://image.slidesharecdn.com/chometabolism-180317164327/85/CHO-metabolism-11-320.jpg)