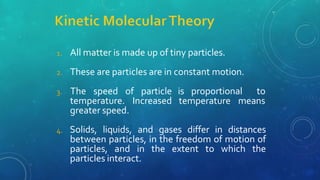
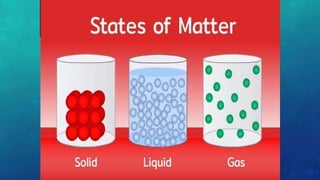
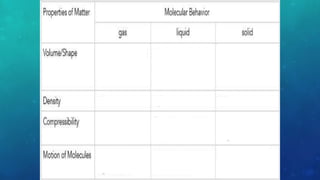
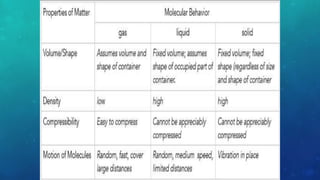
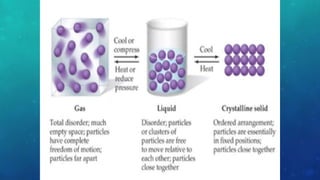
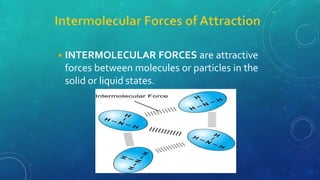
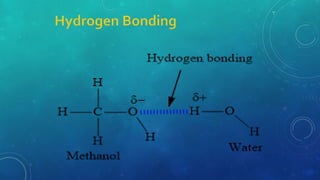
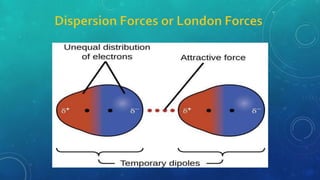
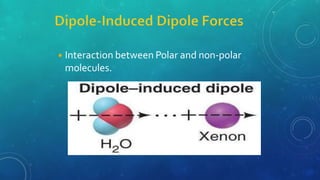
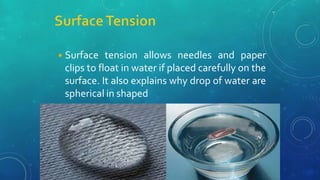
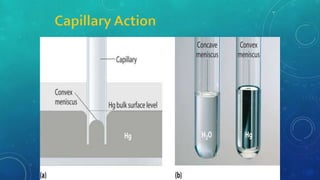
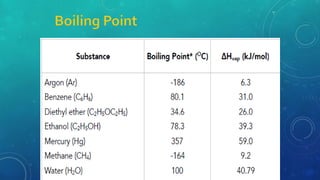
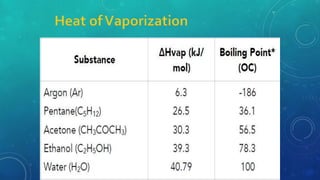
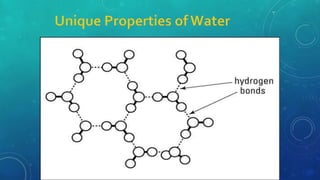
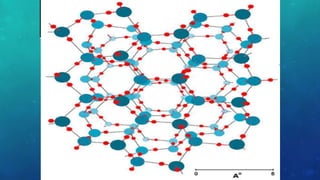
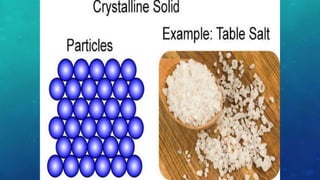
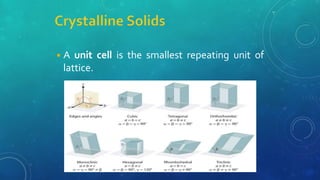
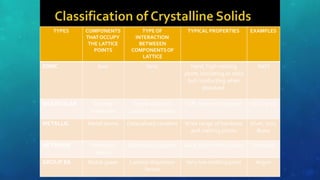
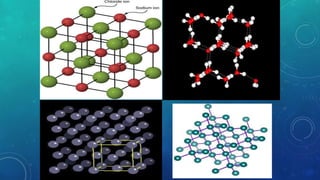
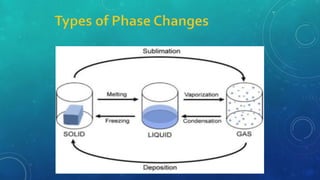
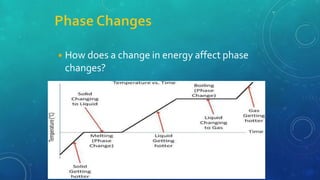
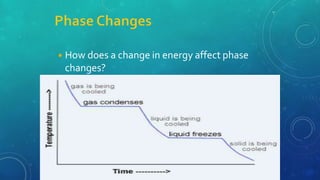
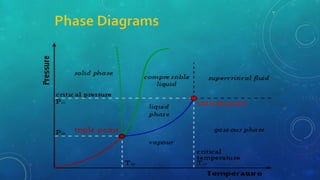
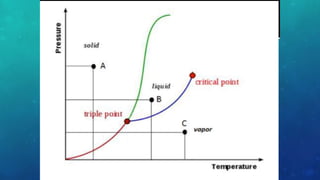
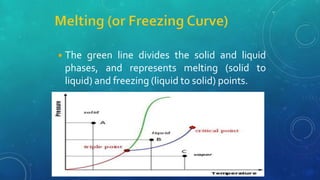
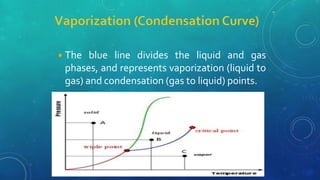
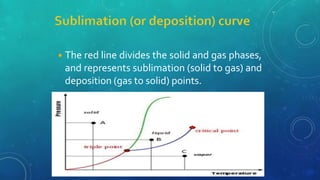
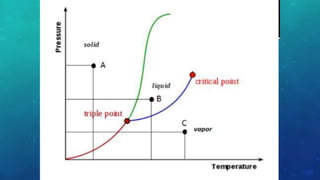
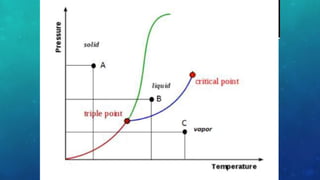
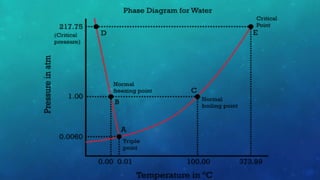
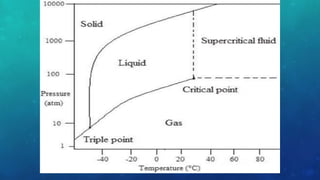
The document discusses the properties of solids and liquids based on the kinetic molecular theory. It explains that solids have a defined structure with particles held closely together by strong intermolecular forces, while liquids lack a defined structure but their properties can be qualitatively understood by considering the intermolecular forces between their particles. The document then discusses several properties of liquids including surface tension, viscosity, vapor pressure, boiling point, and heat of vaporization, relating them to the intermolecular forces between the liquid particles.