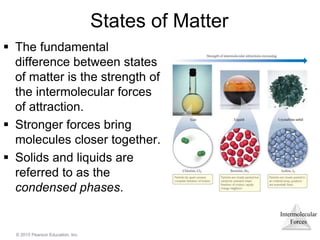
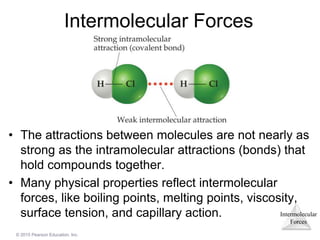
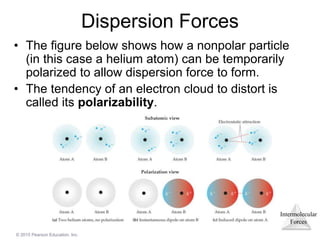
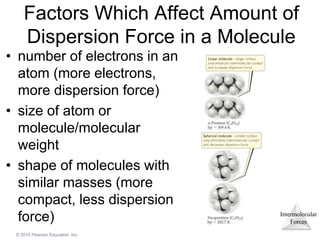
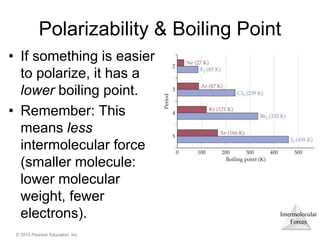
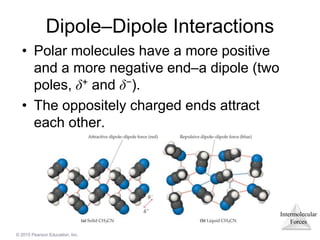
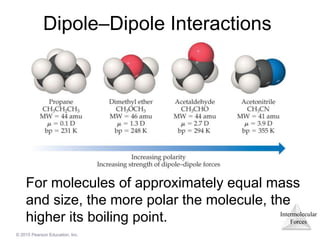
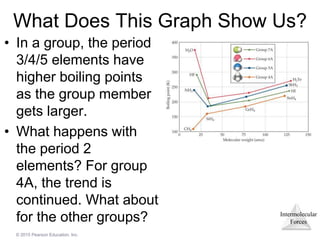
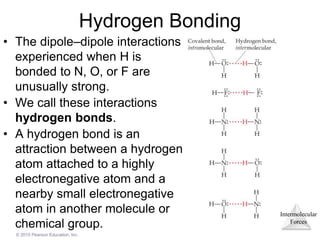
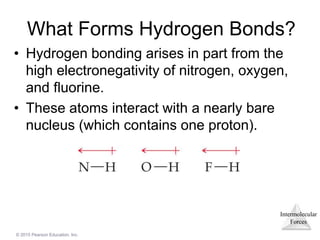
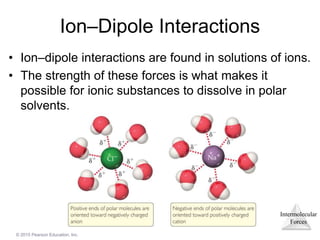
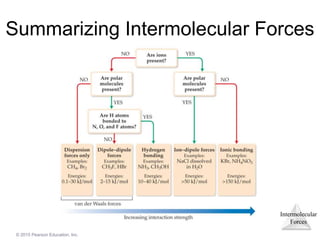
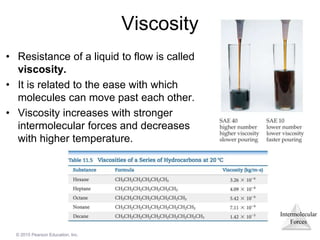
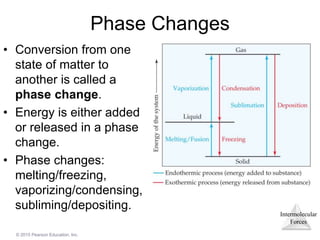
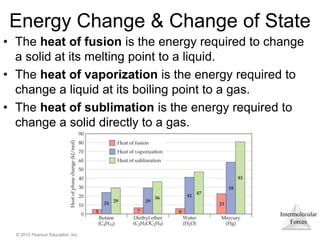
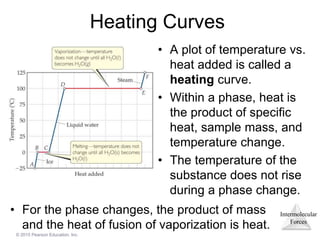
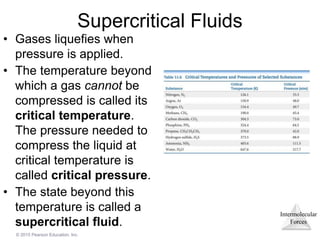
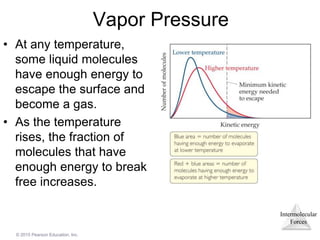
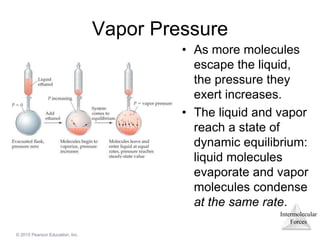
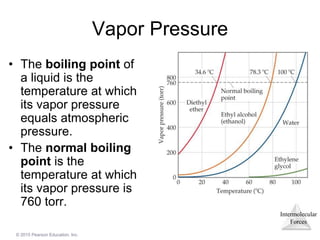
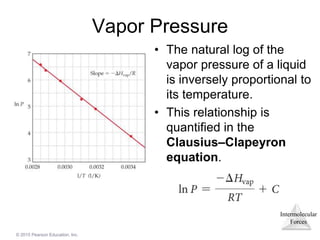
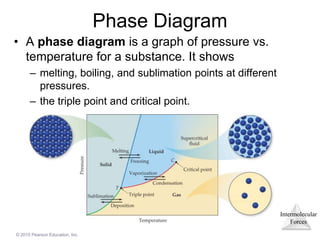
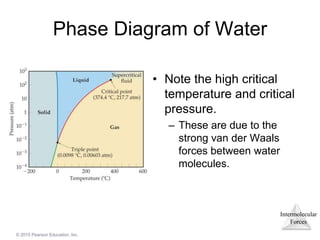
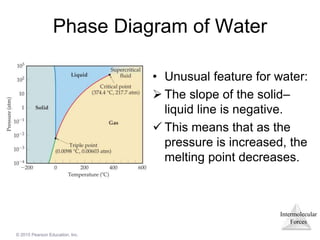
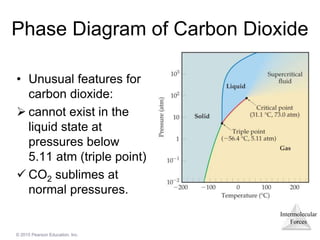
The document discusses intermolecular forces and how they influence the properties of liquids. It explains that the strength of intermolecular forces determines whether a substance is a solid, liquid or gas. The main intermolecular forces are dispersion forces, dipole-dipole interactions, hydrogen bonding, and ion-dipole interactions. Stronger intermolecular forces result in higher boiling points and melting points, as well as properties like viscosity, surface tension and capillary action. Phase changes between solid, liquid and gas require energy and are influenced by intermolecular forces.