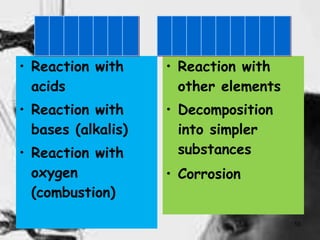
This document discusses physical and chemical changes in matter. It defines physical changes as changes in state or form without a change in chemical composition, such as water freezing to ice. Chemical changes result in new substances forming through chemical reactions, as seen when iron rusts. The document outlines several properties and signs that can indicate if a physical or chemical change has occurred, such as changes in density, melting point, or the production of gas.