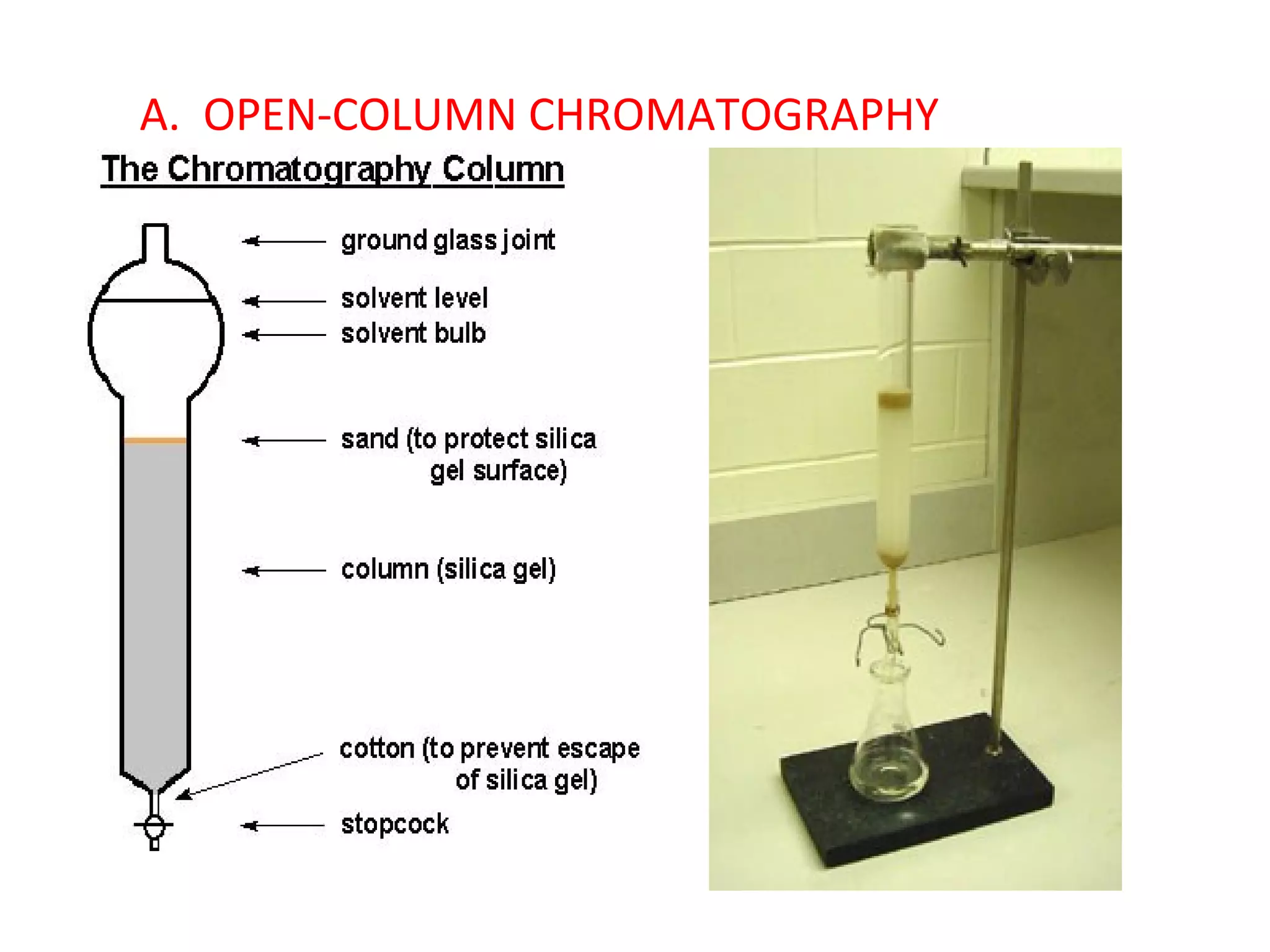
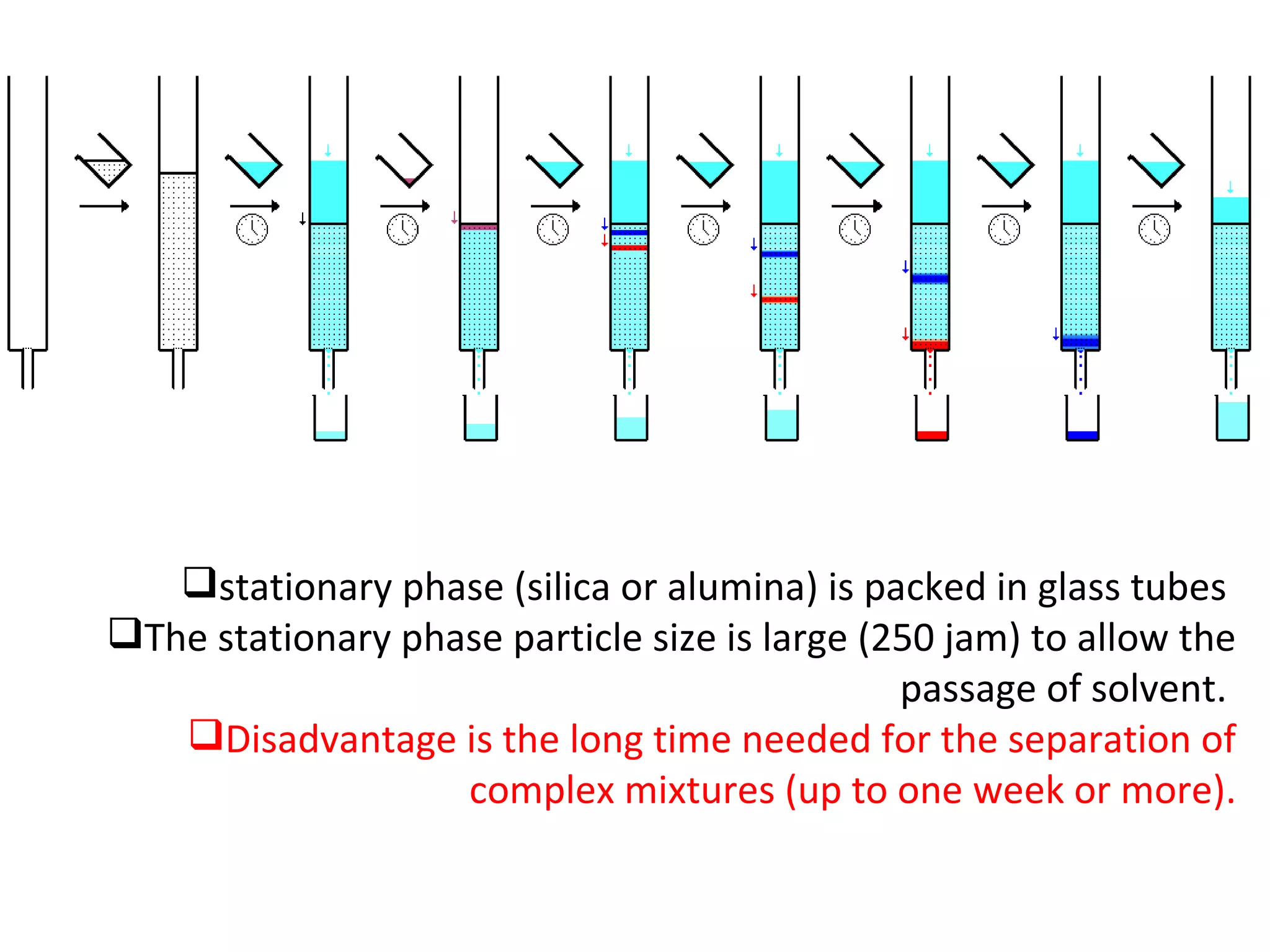
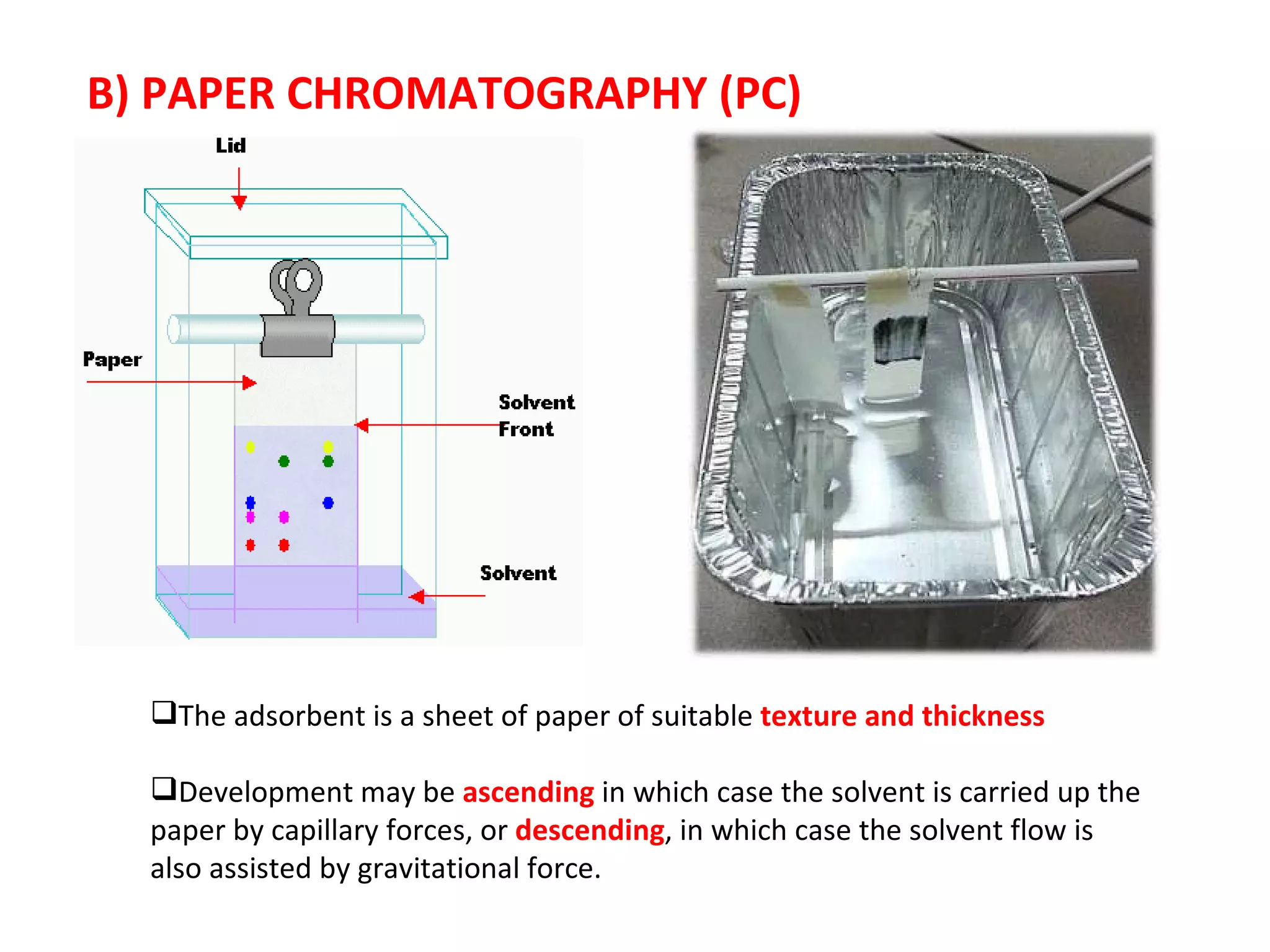
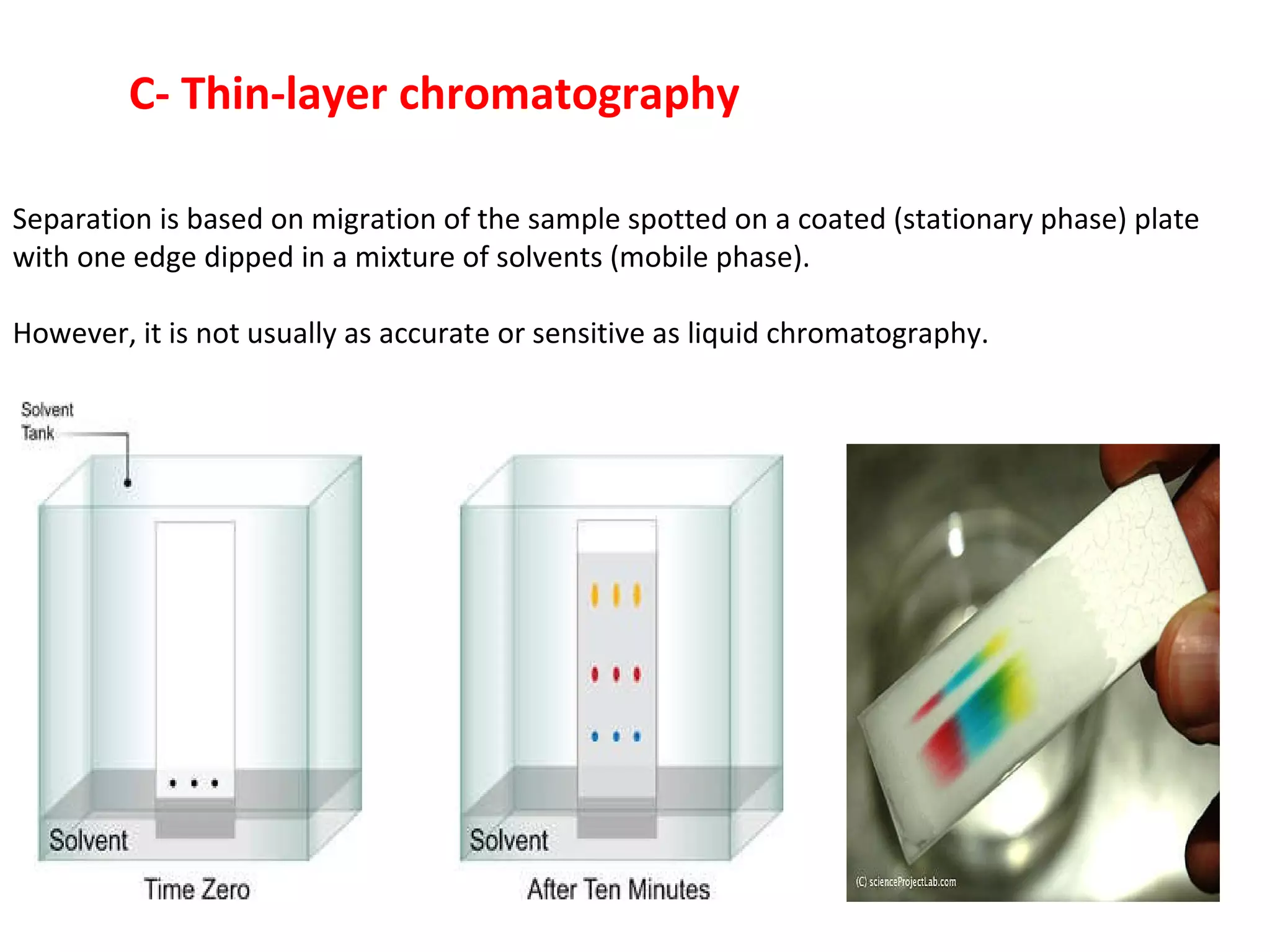
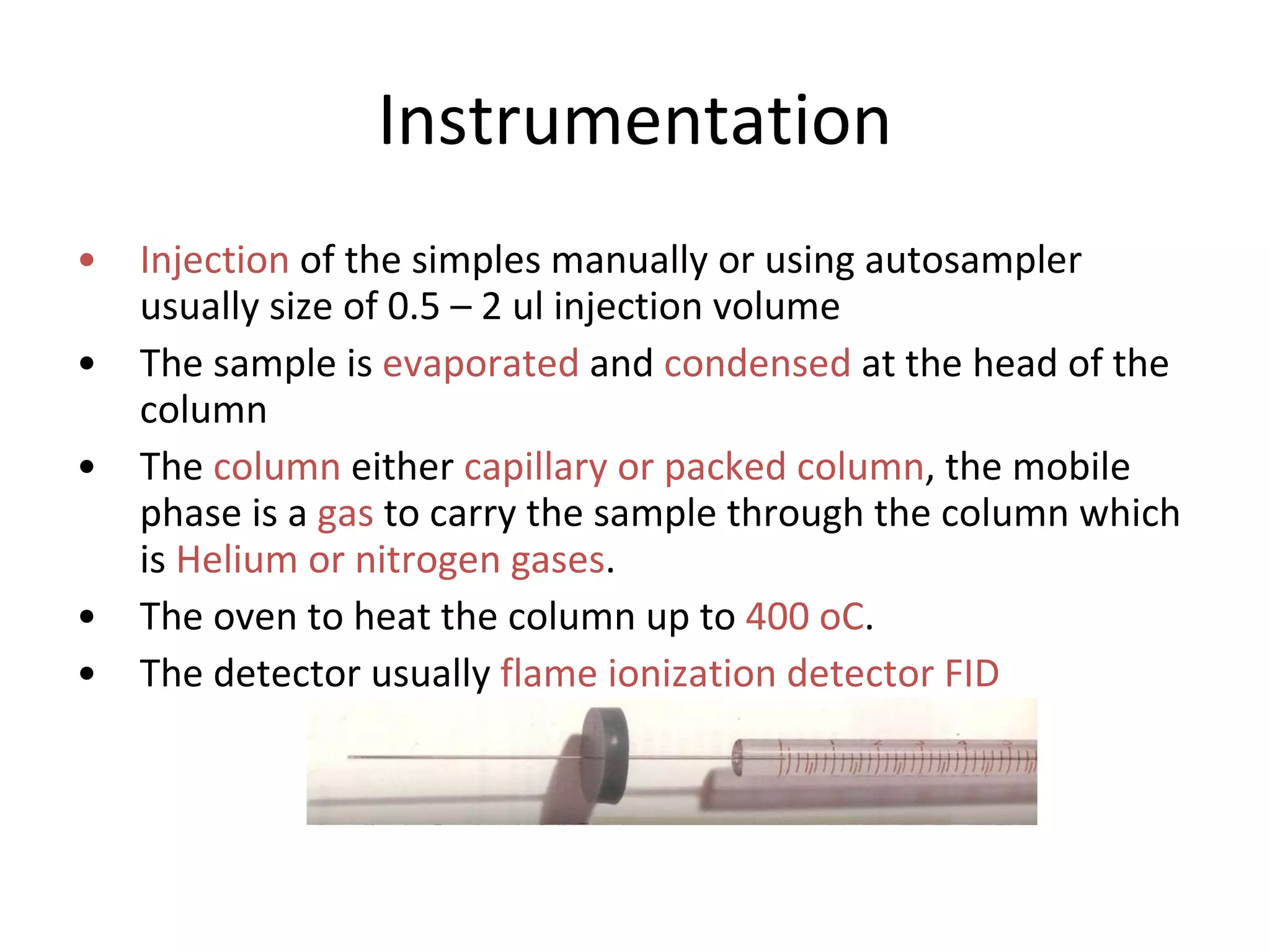
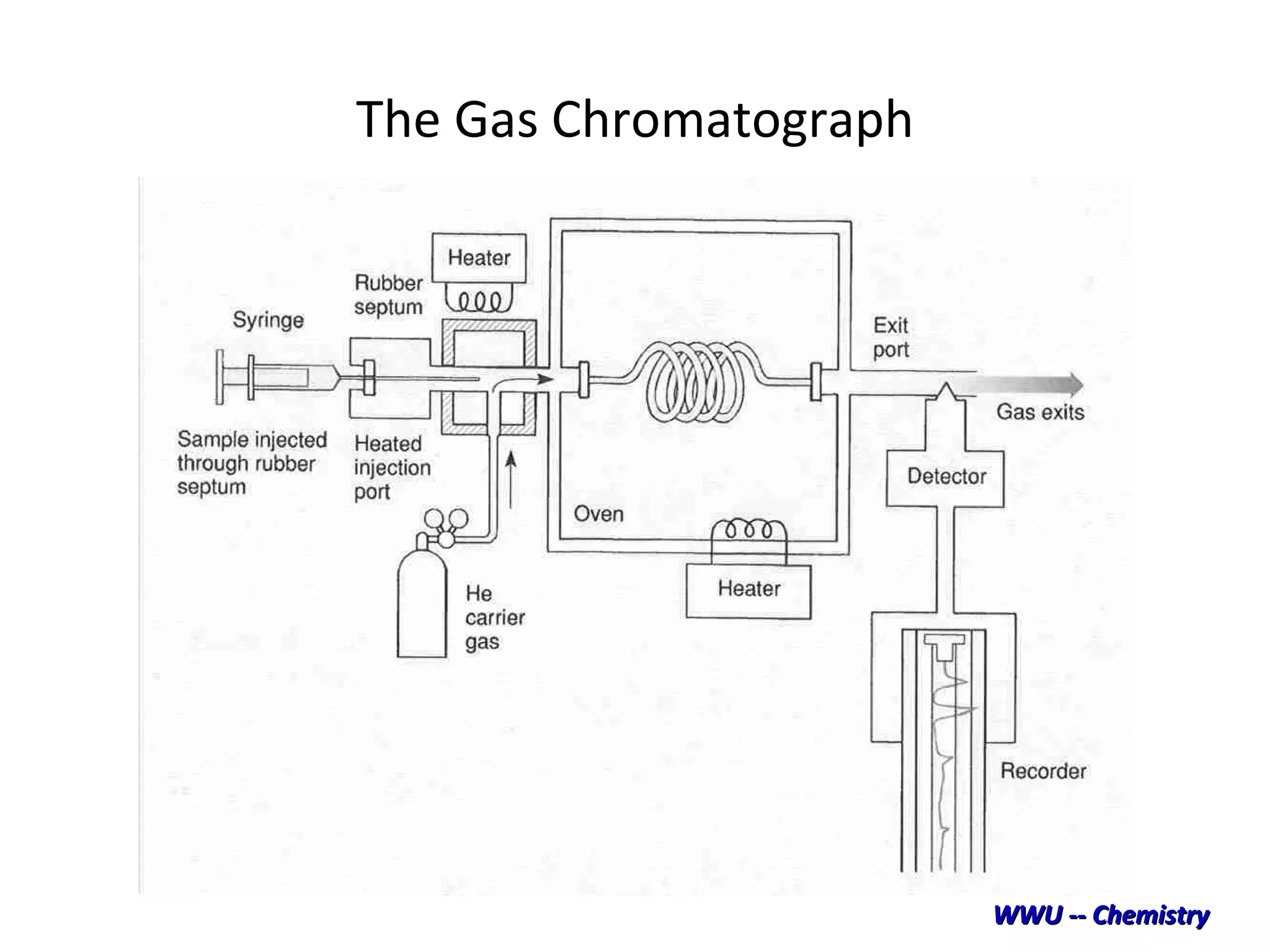
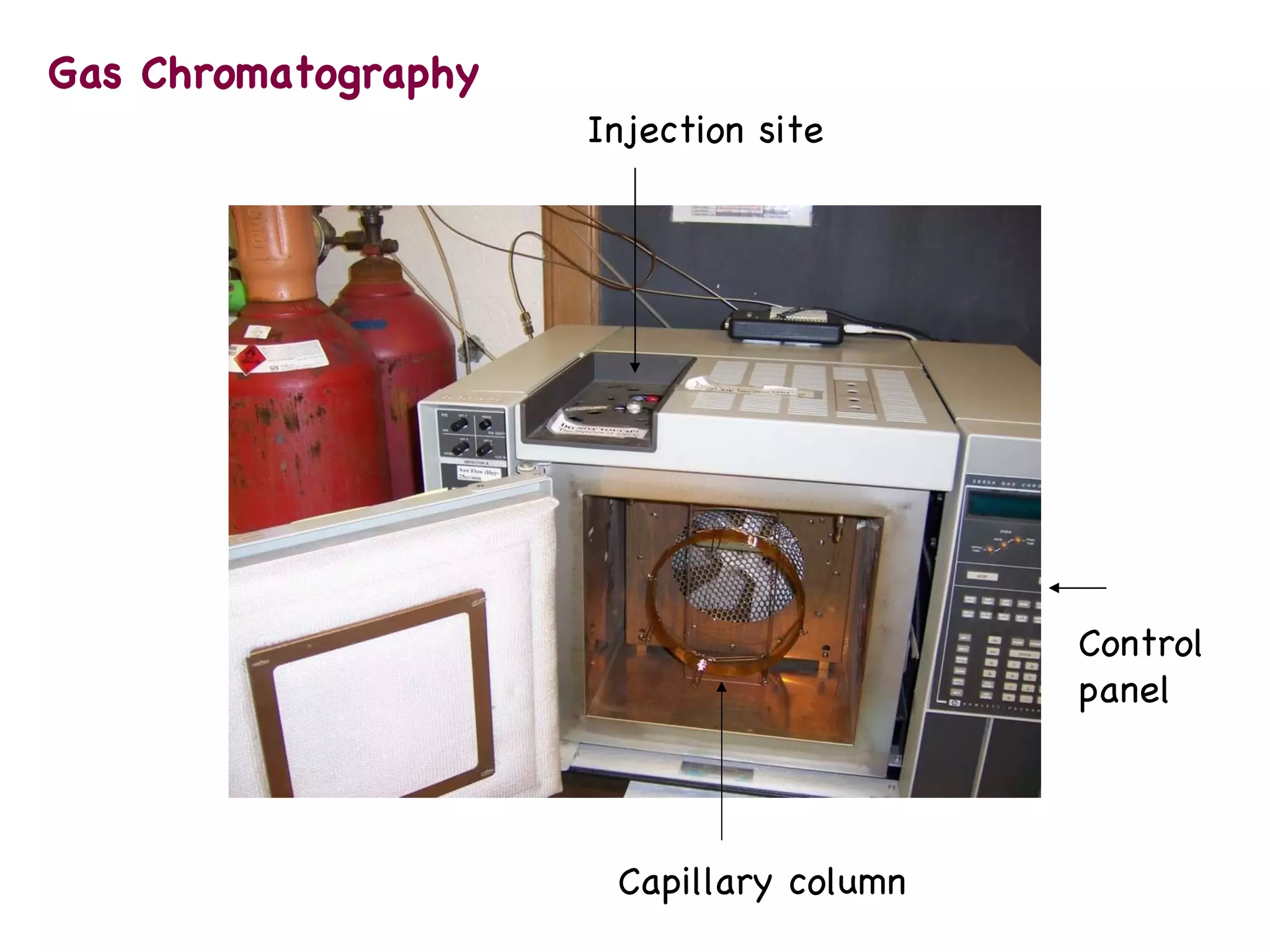
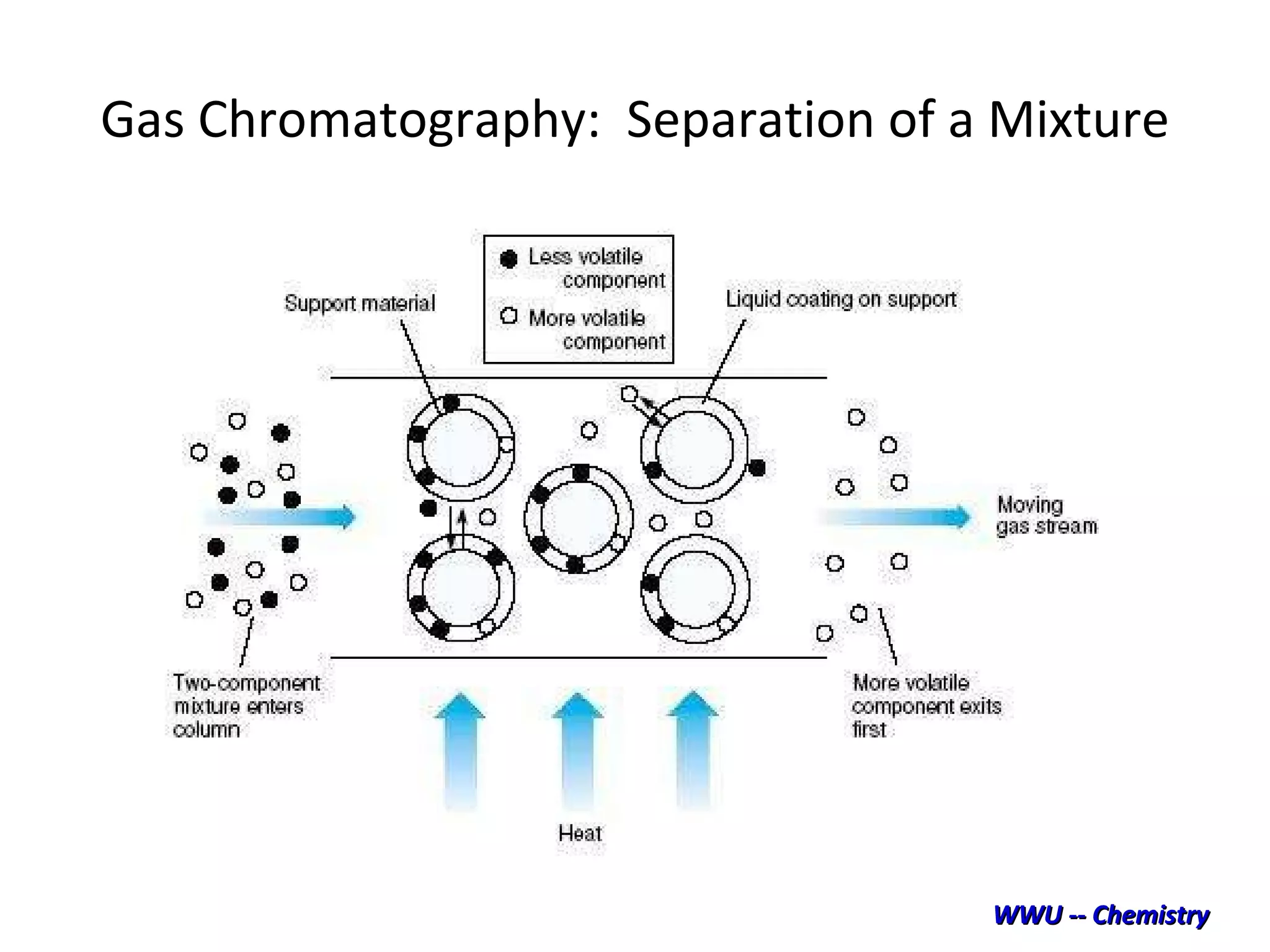
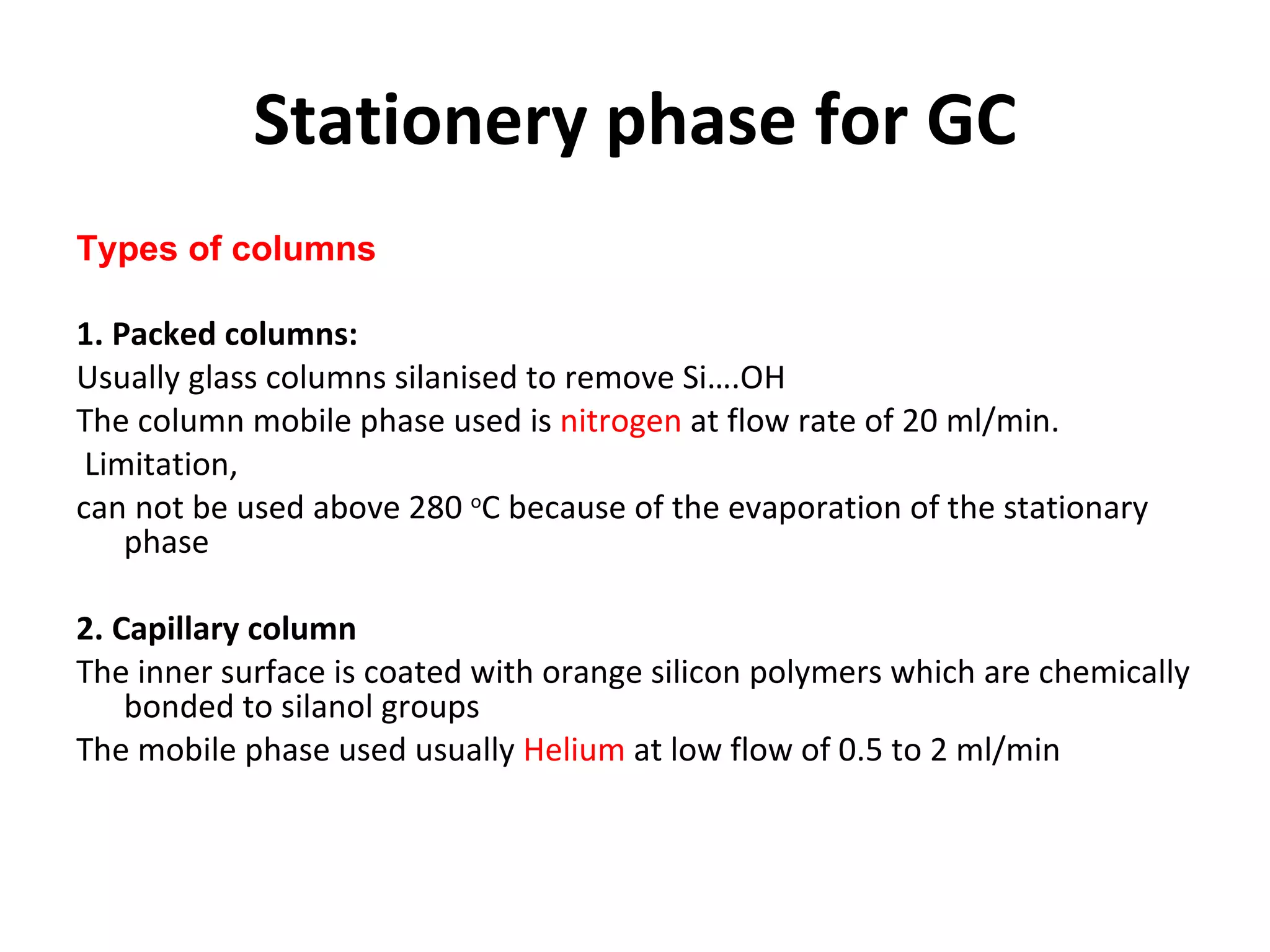
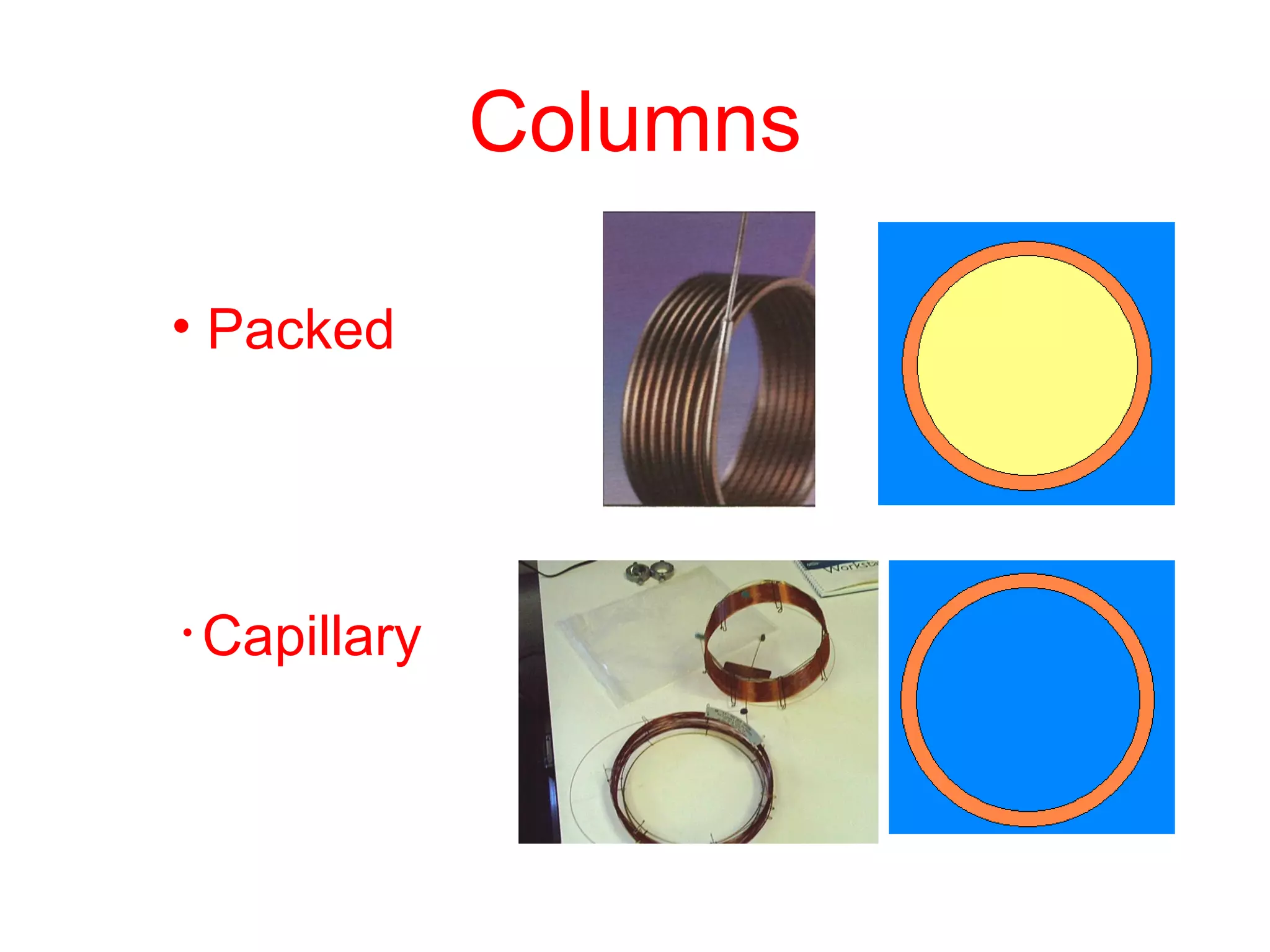
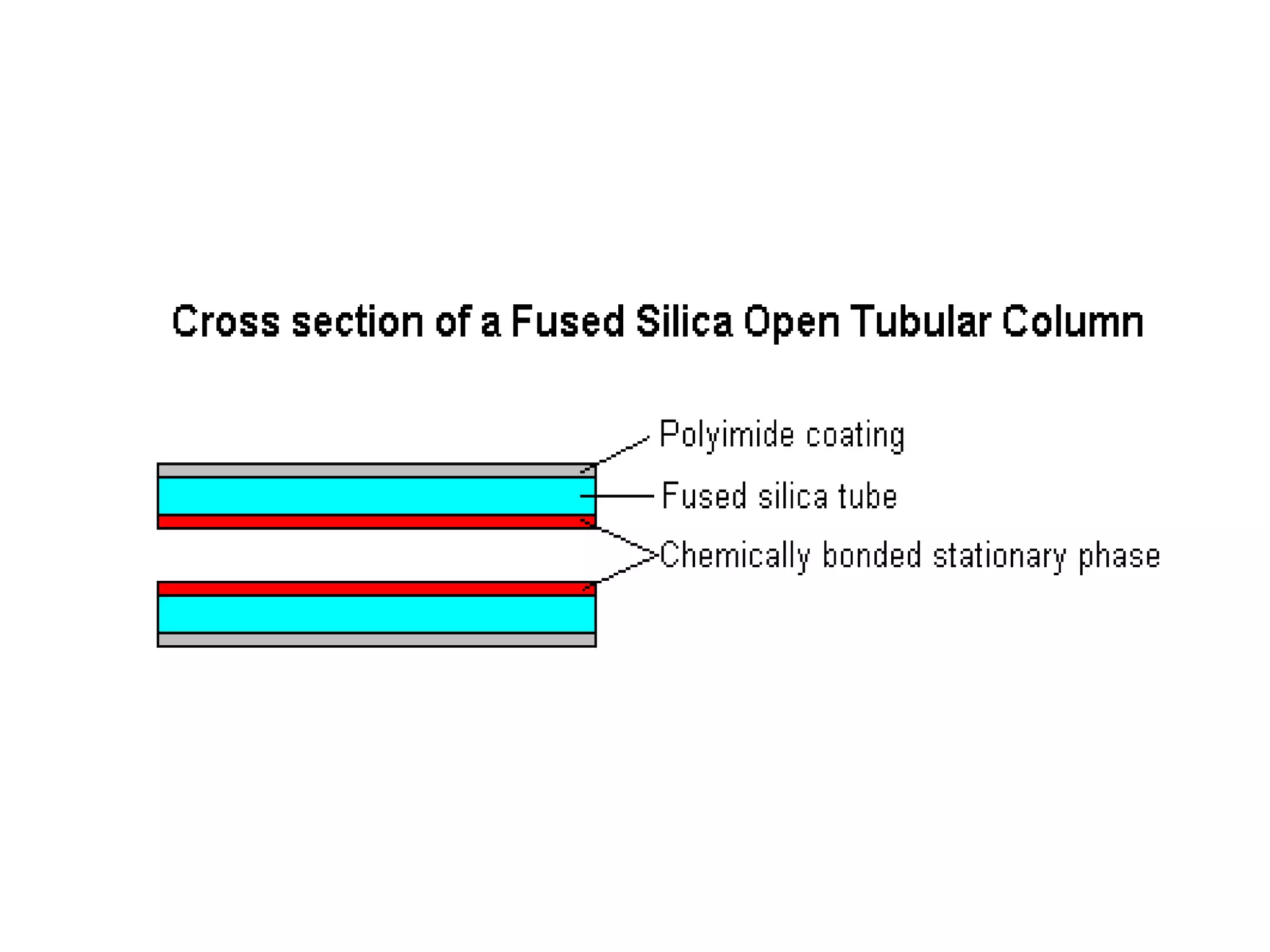
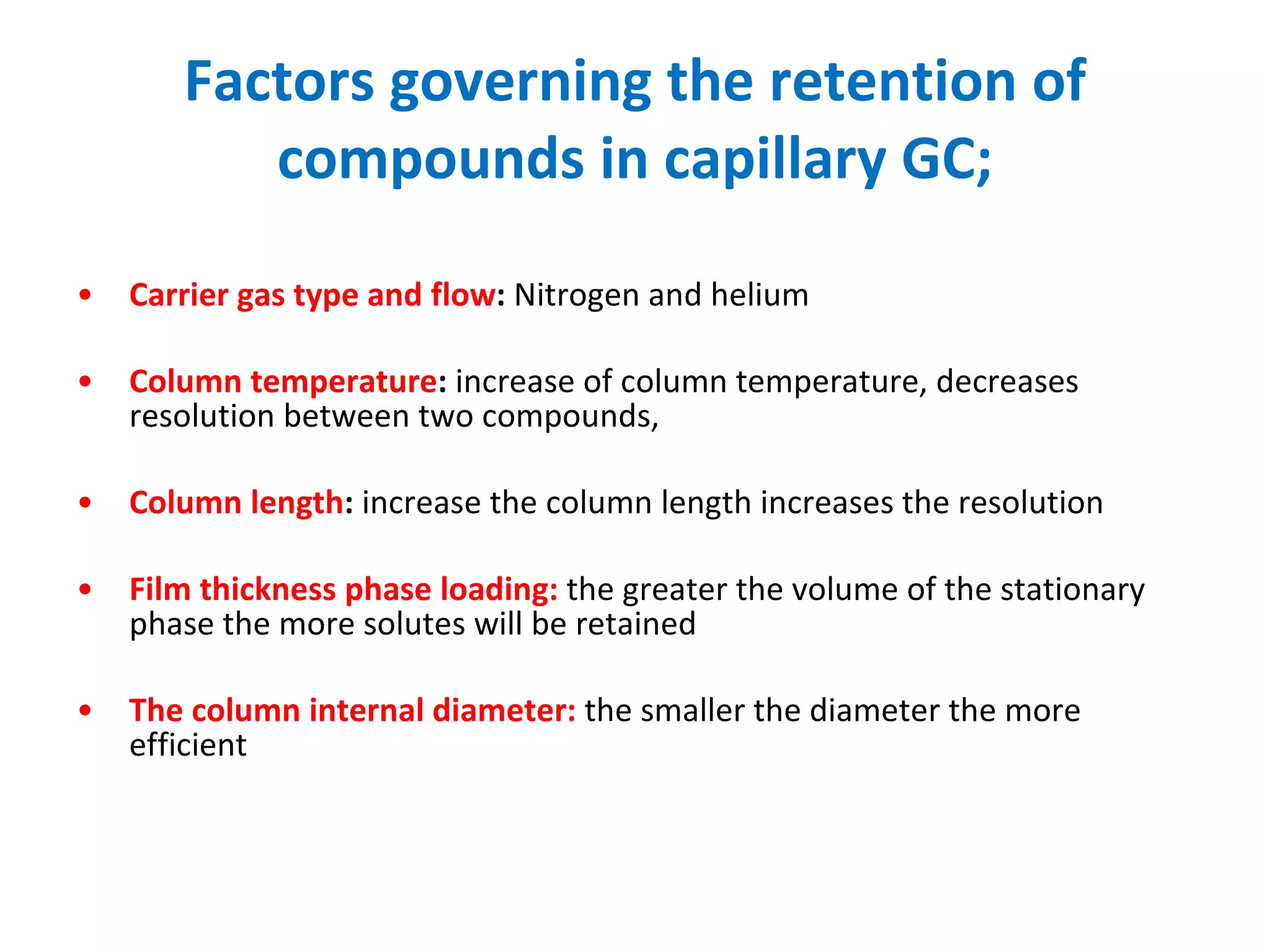
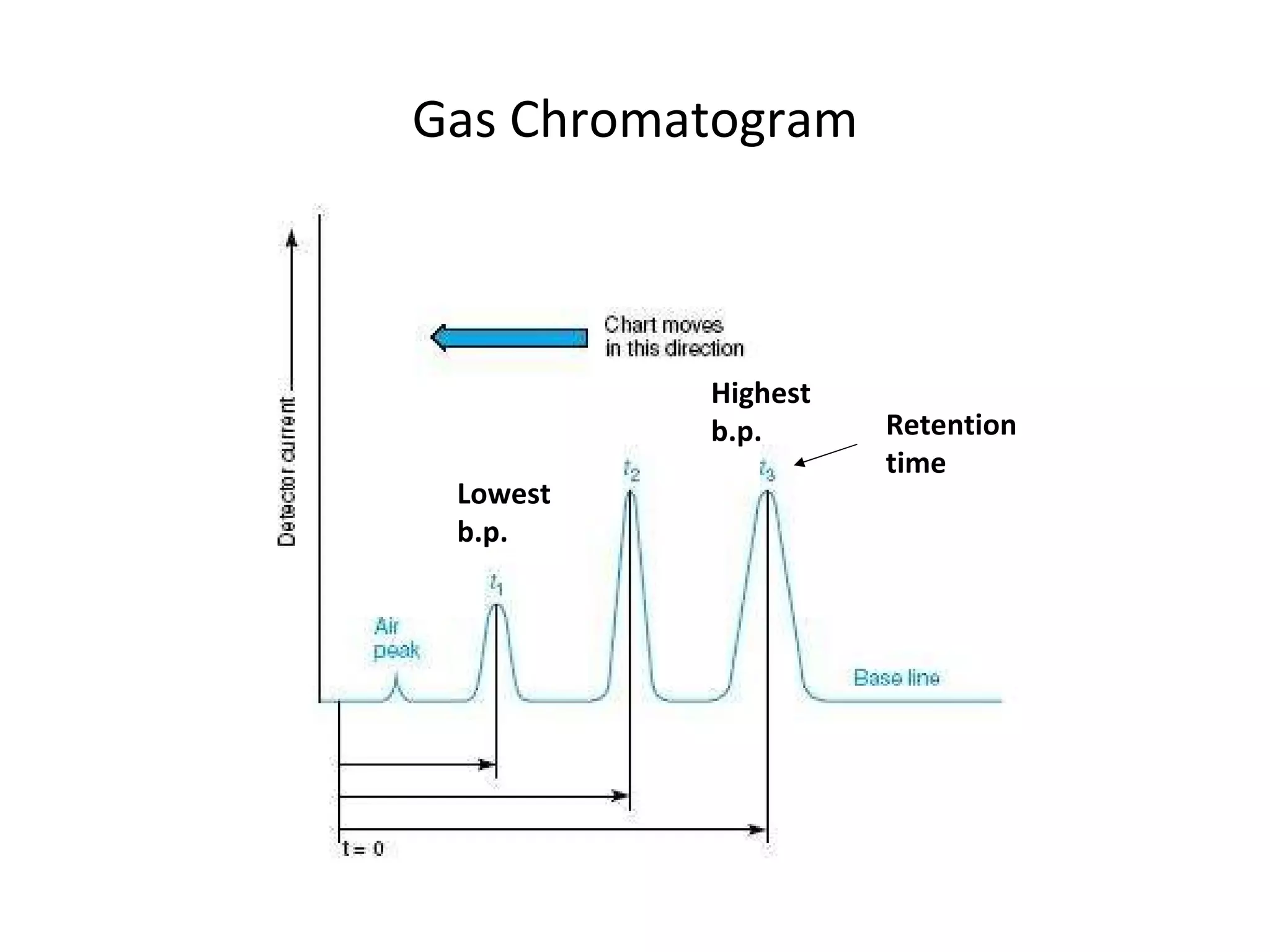
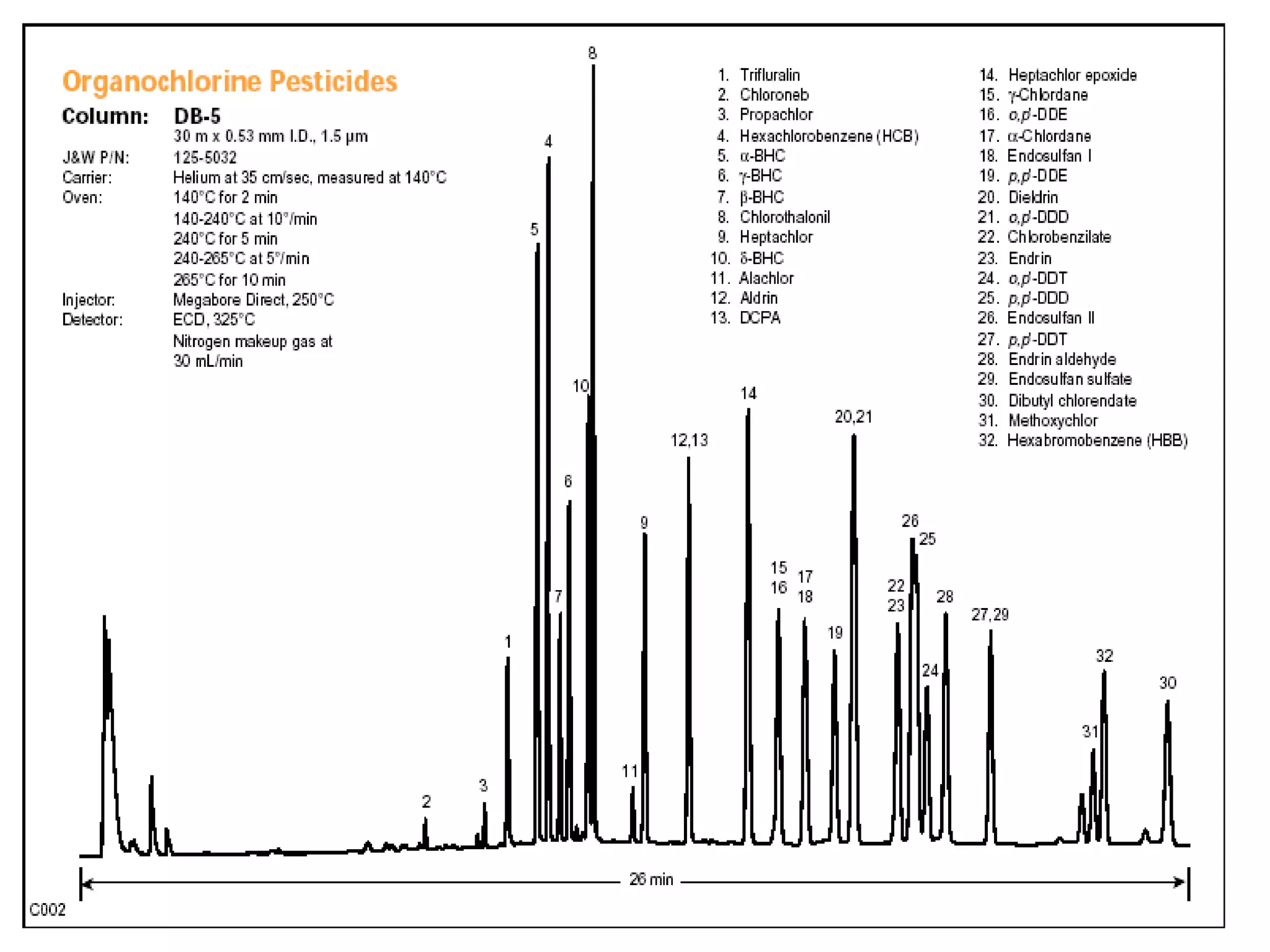
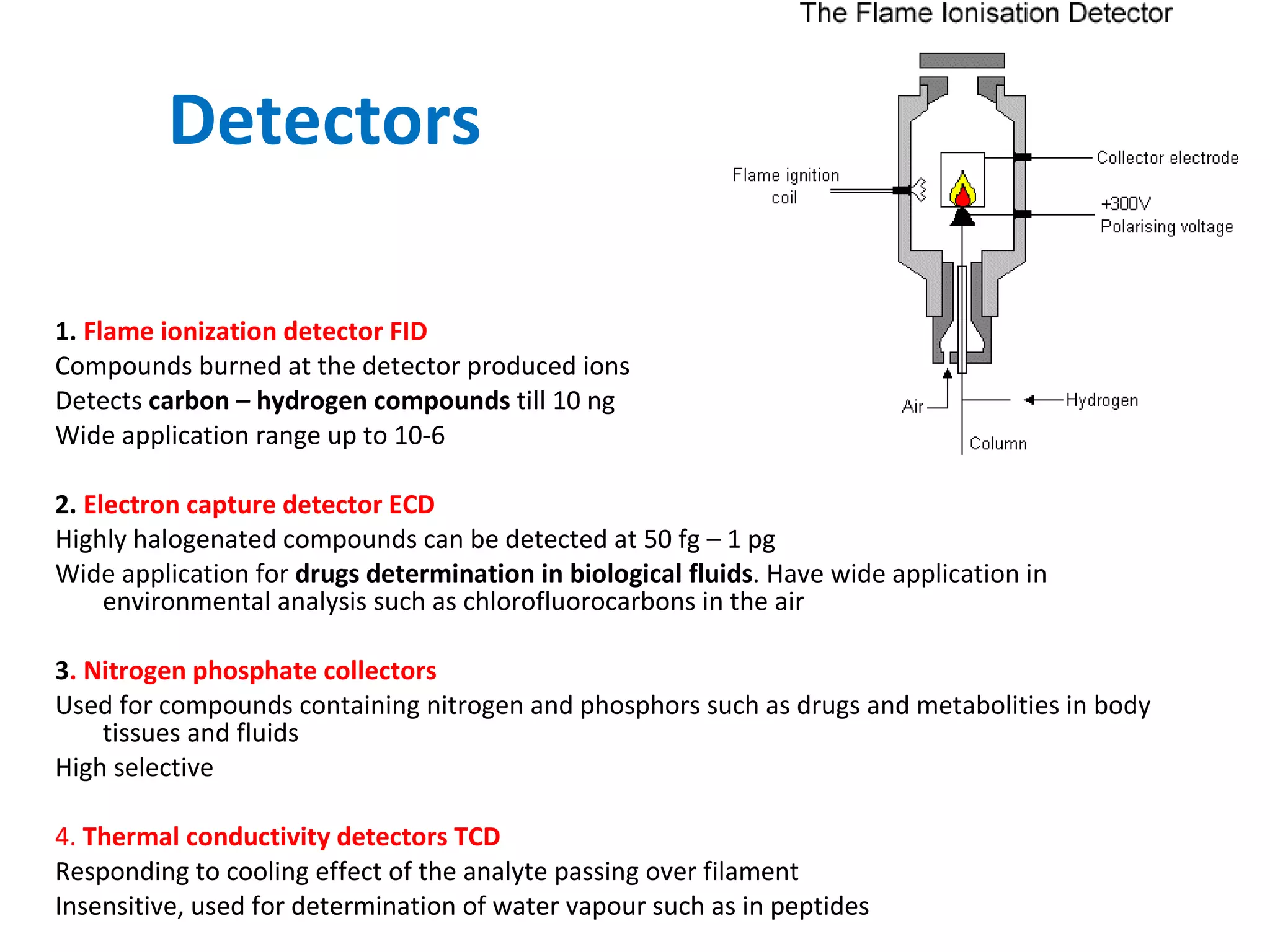
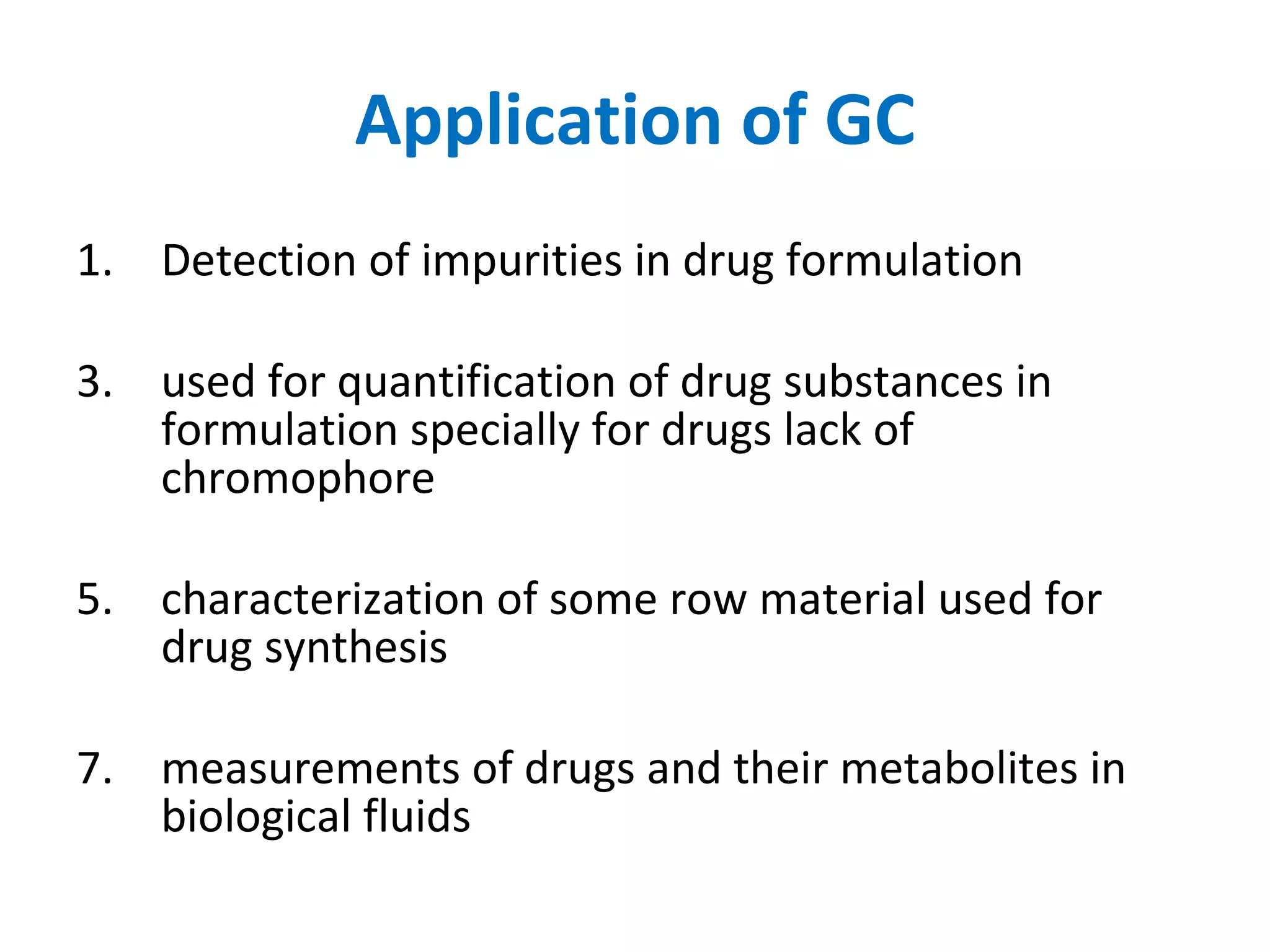
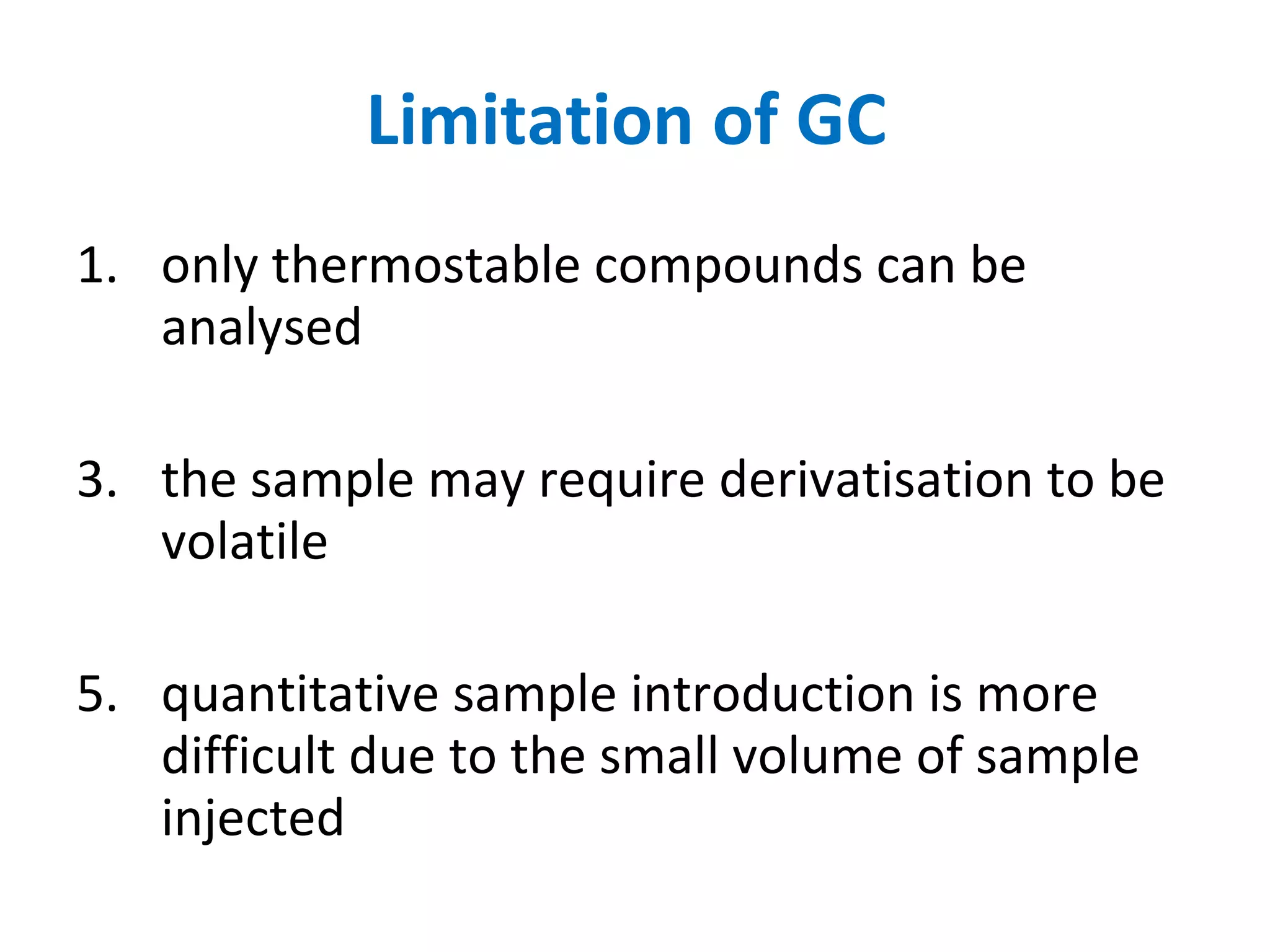
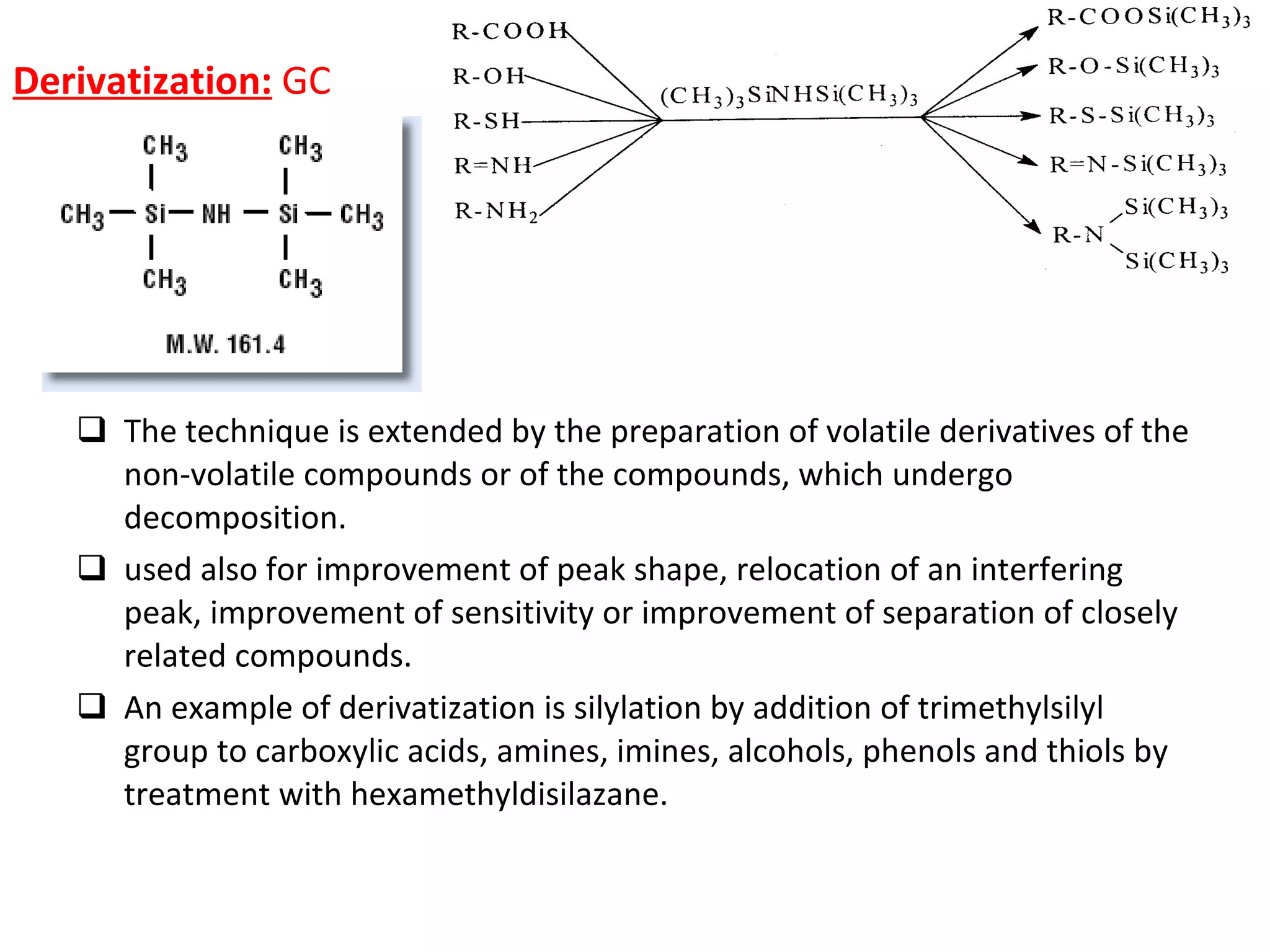
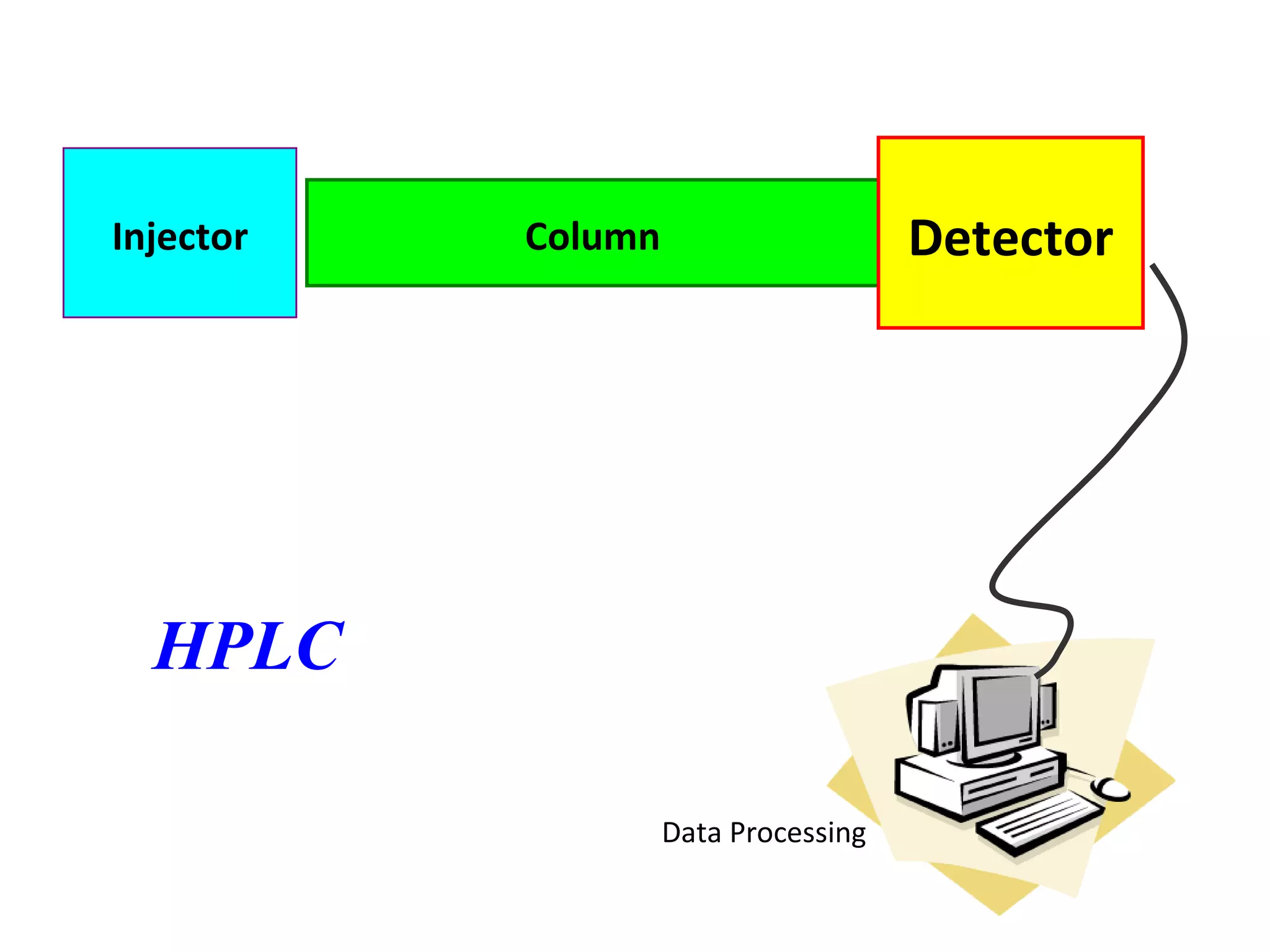
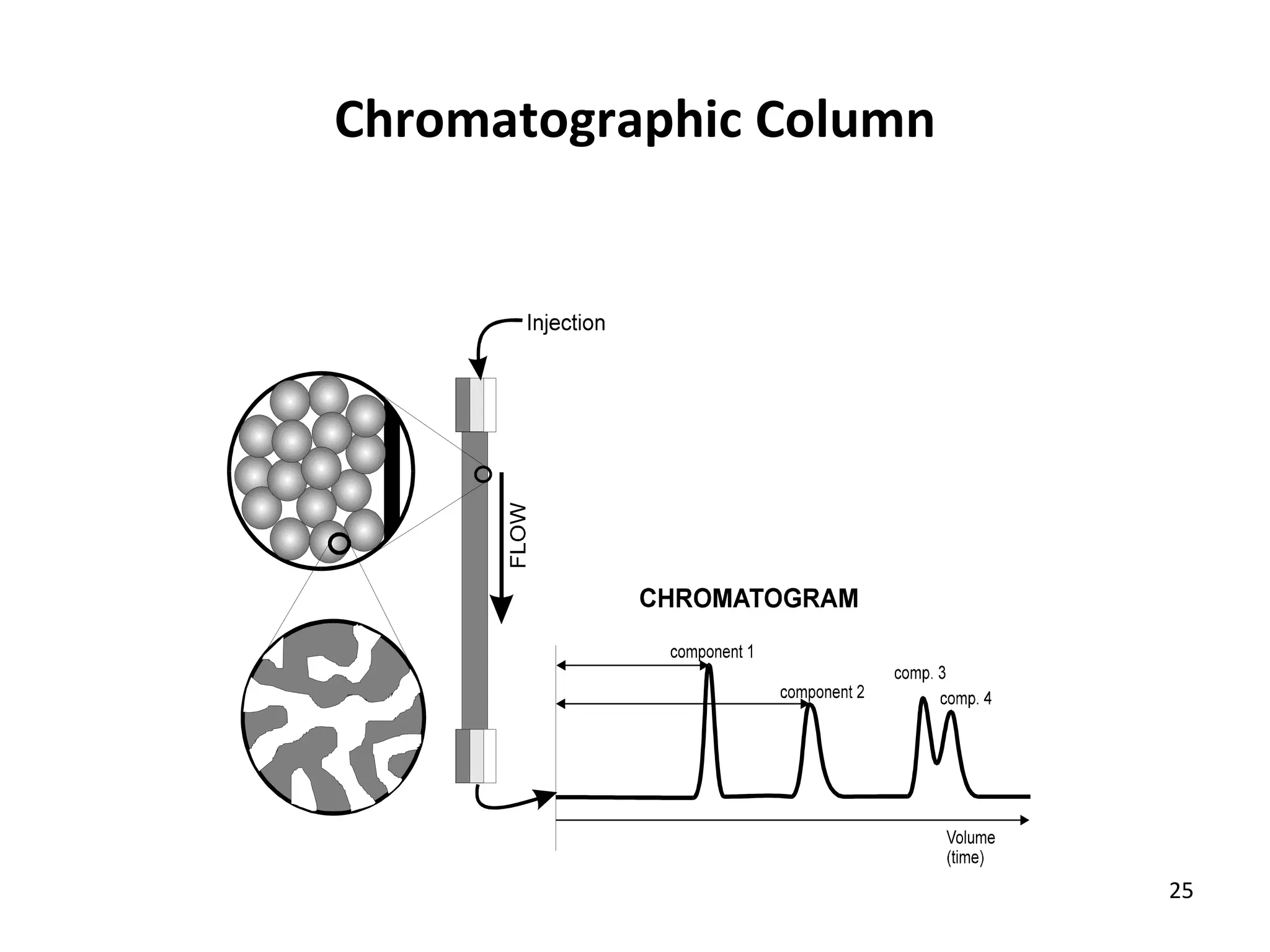
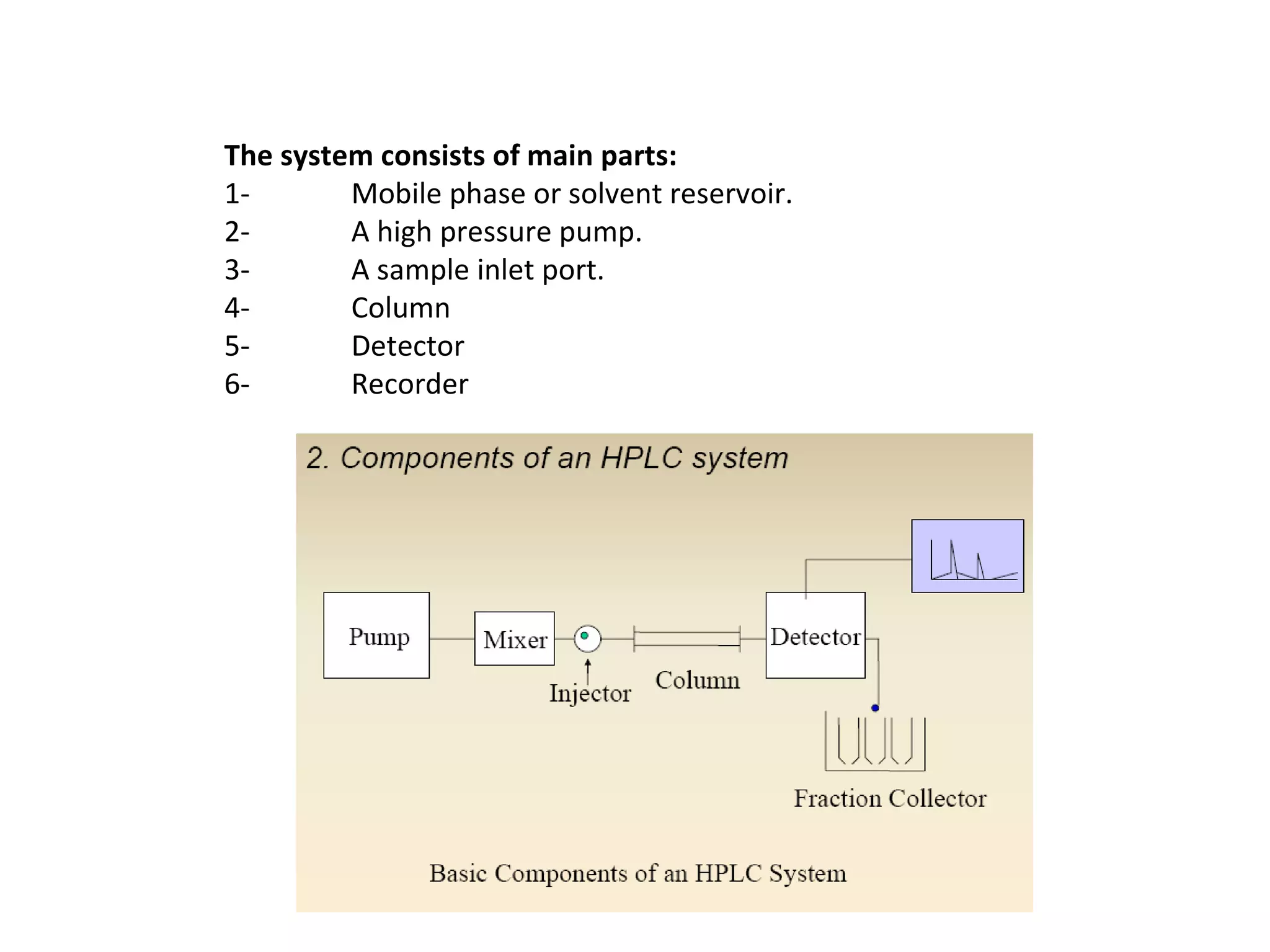
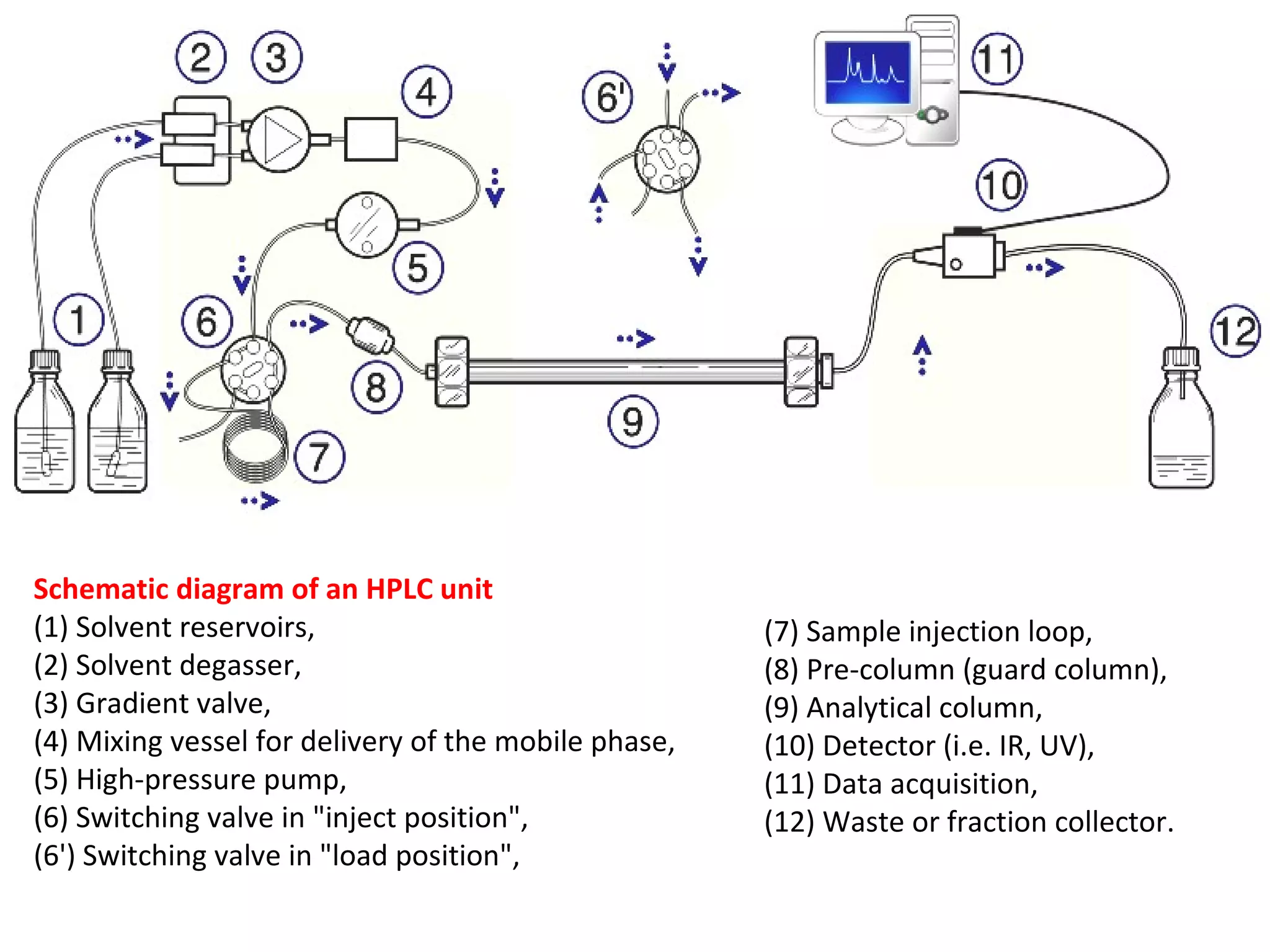
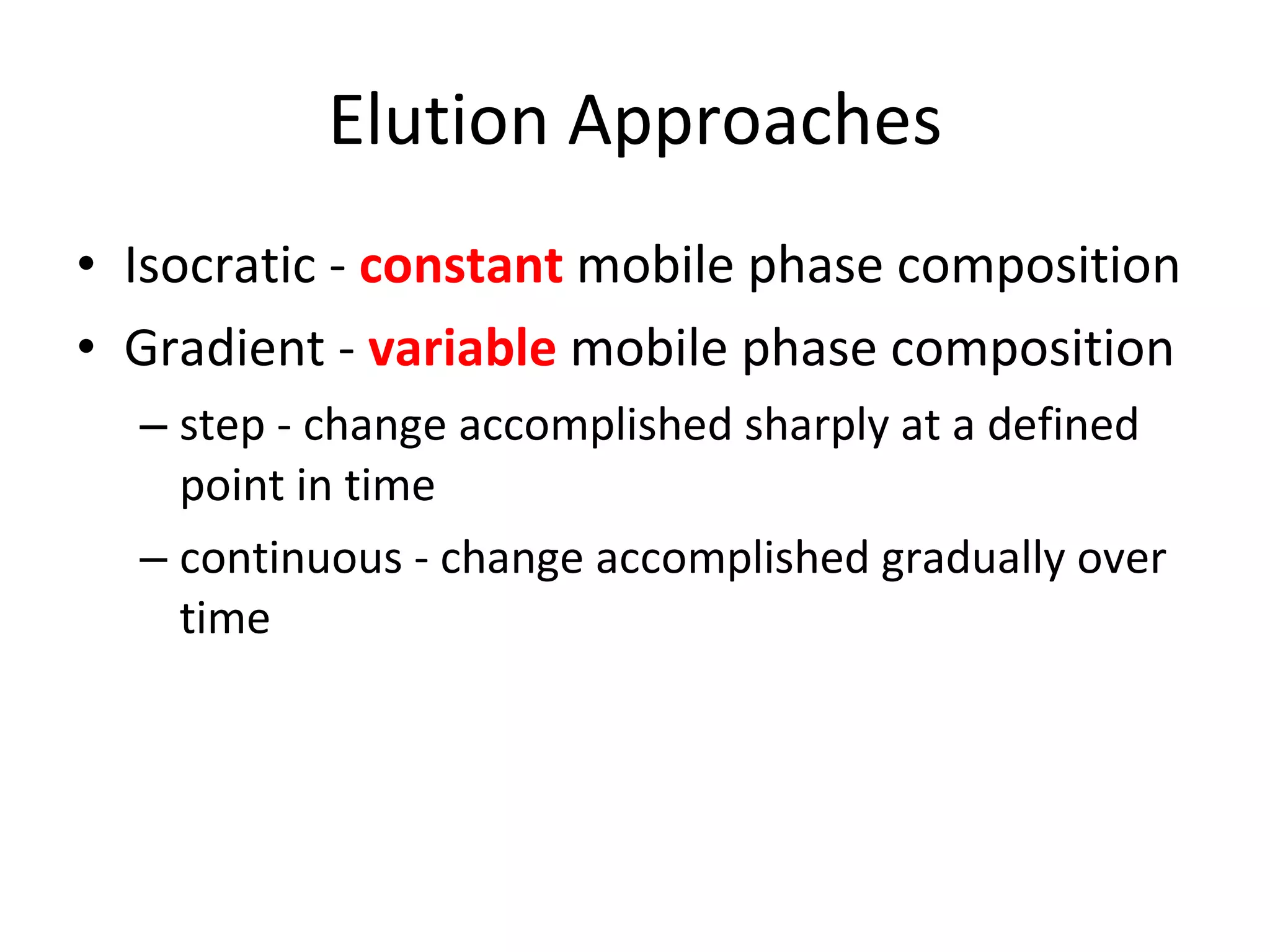
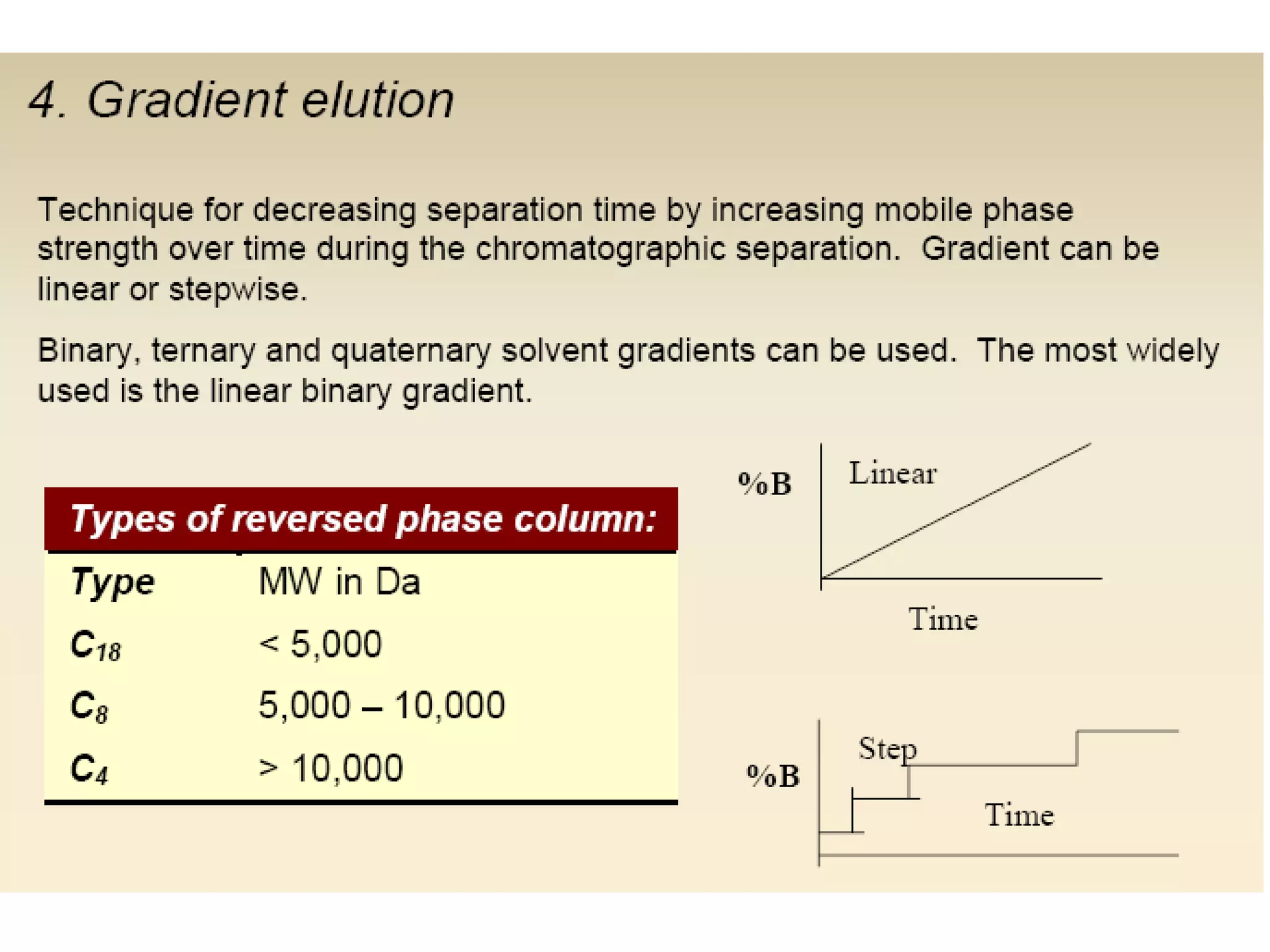
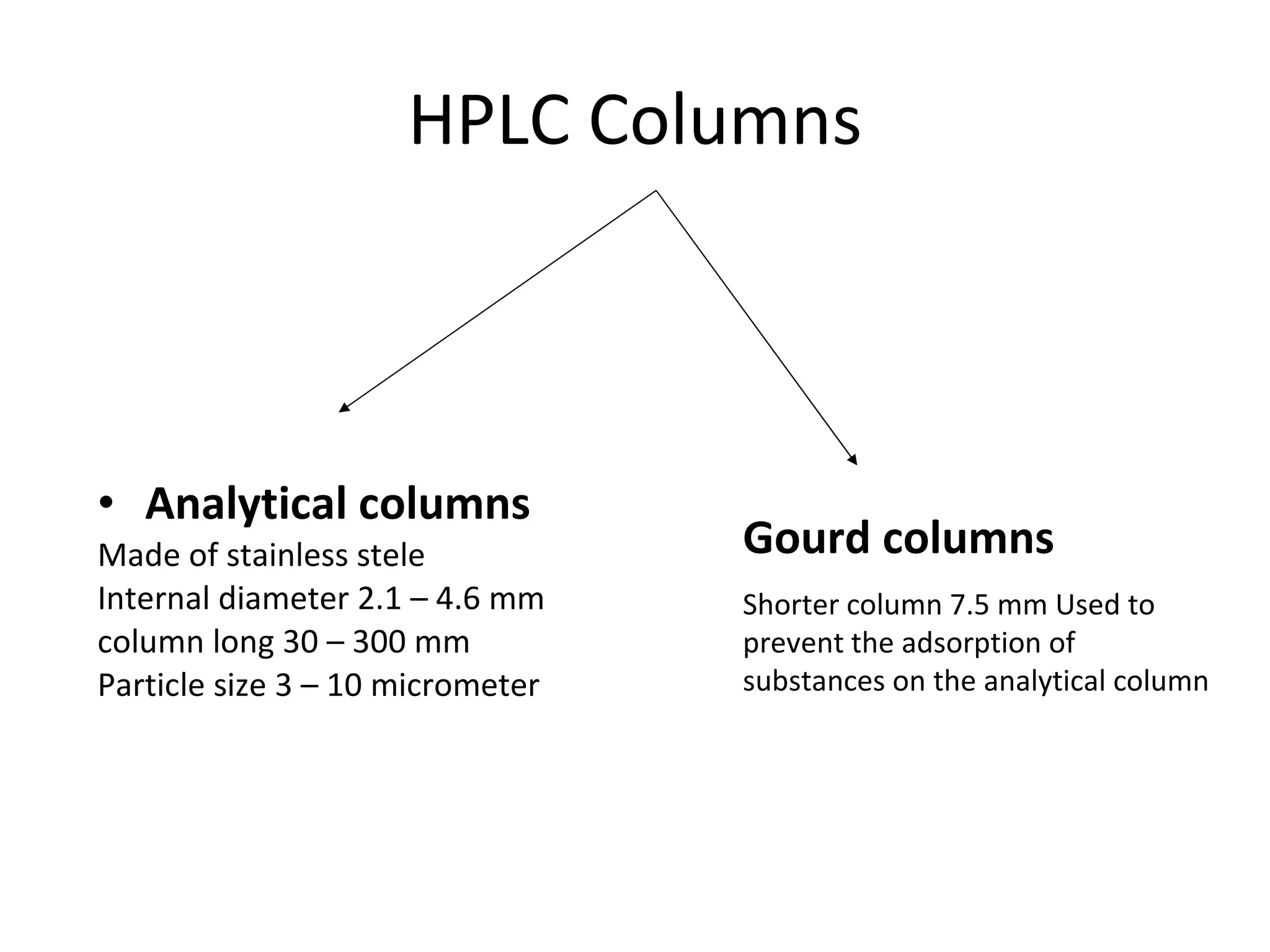
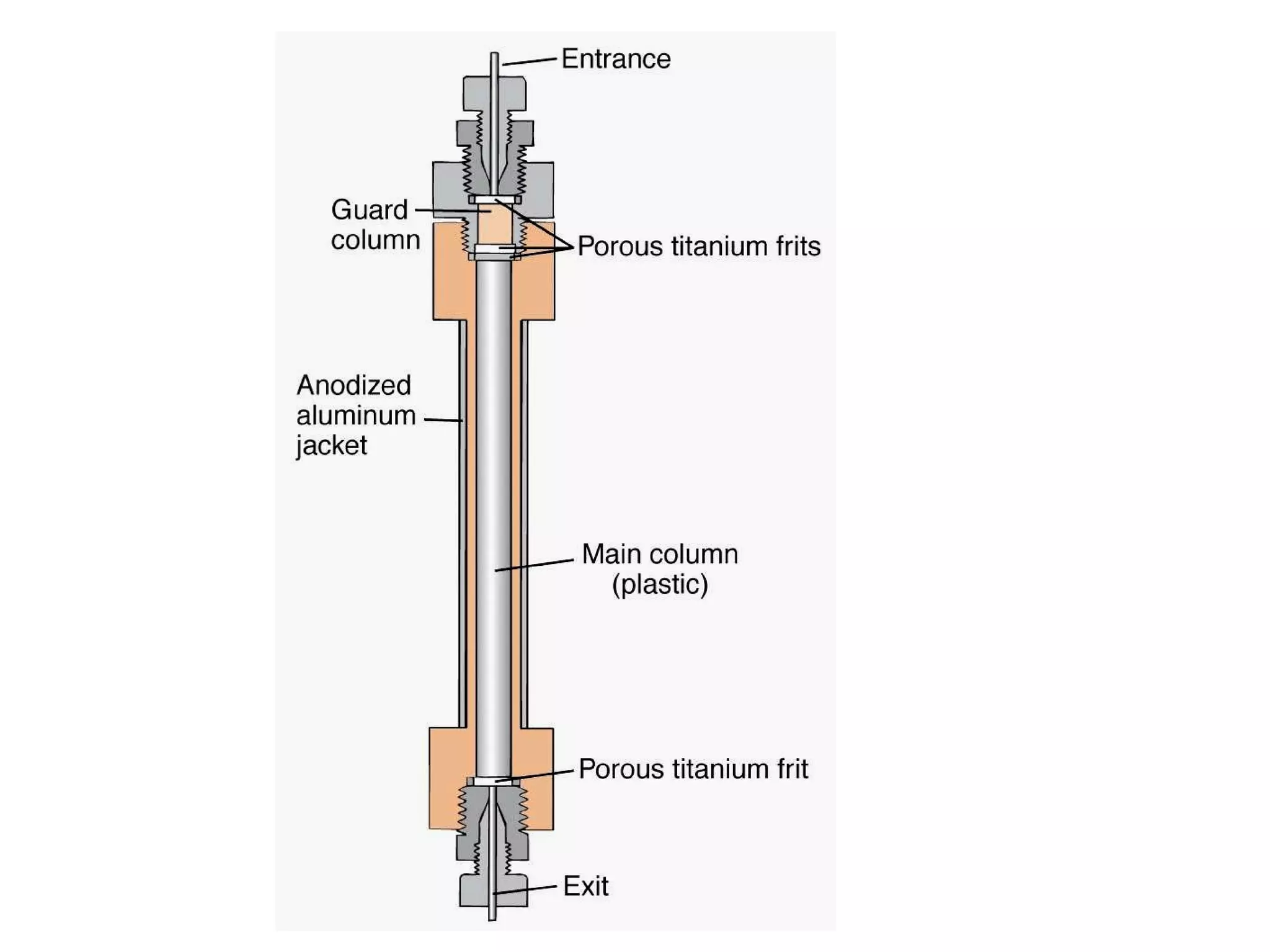
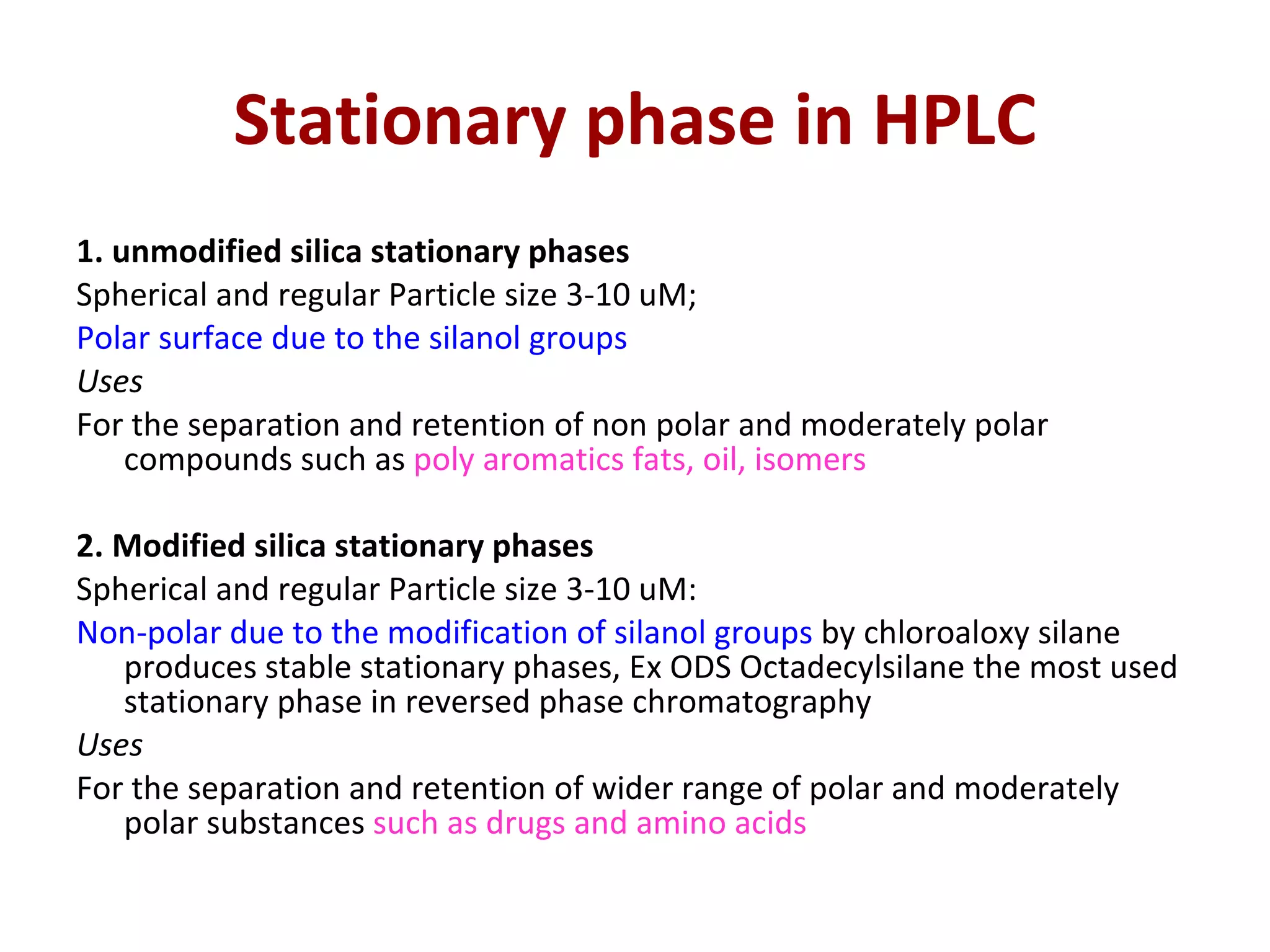
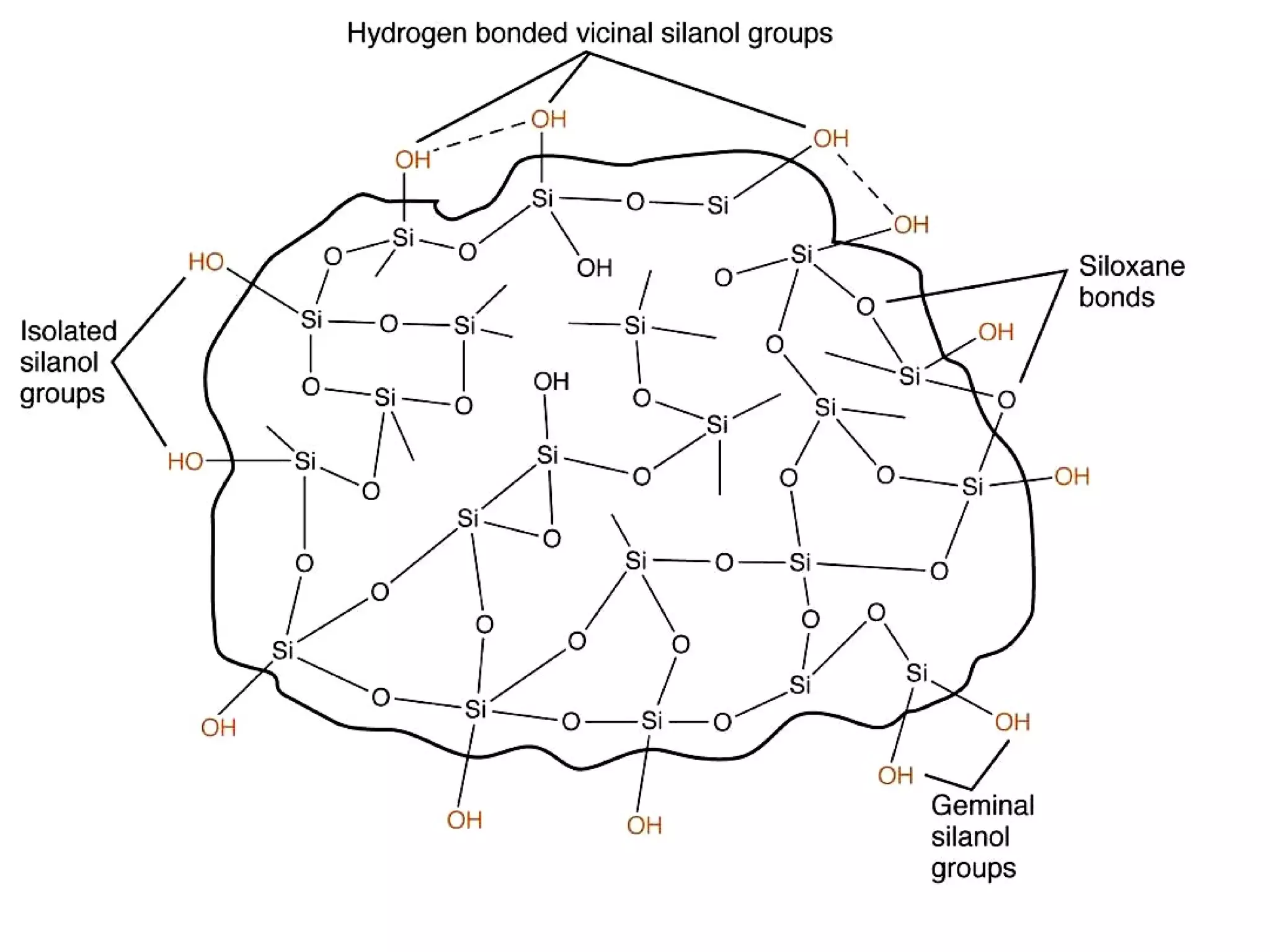
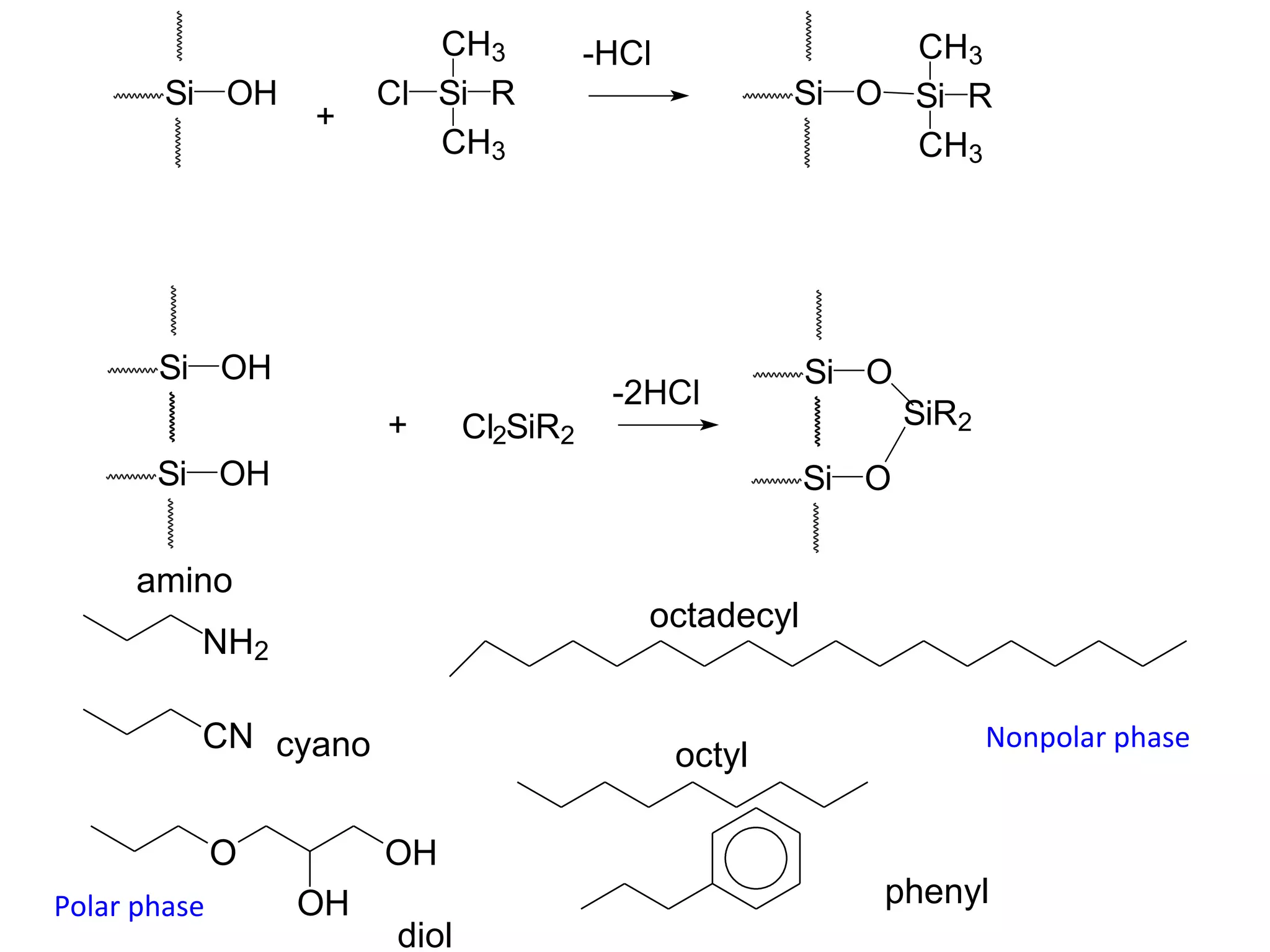
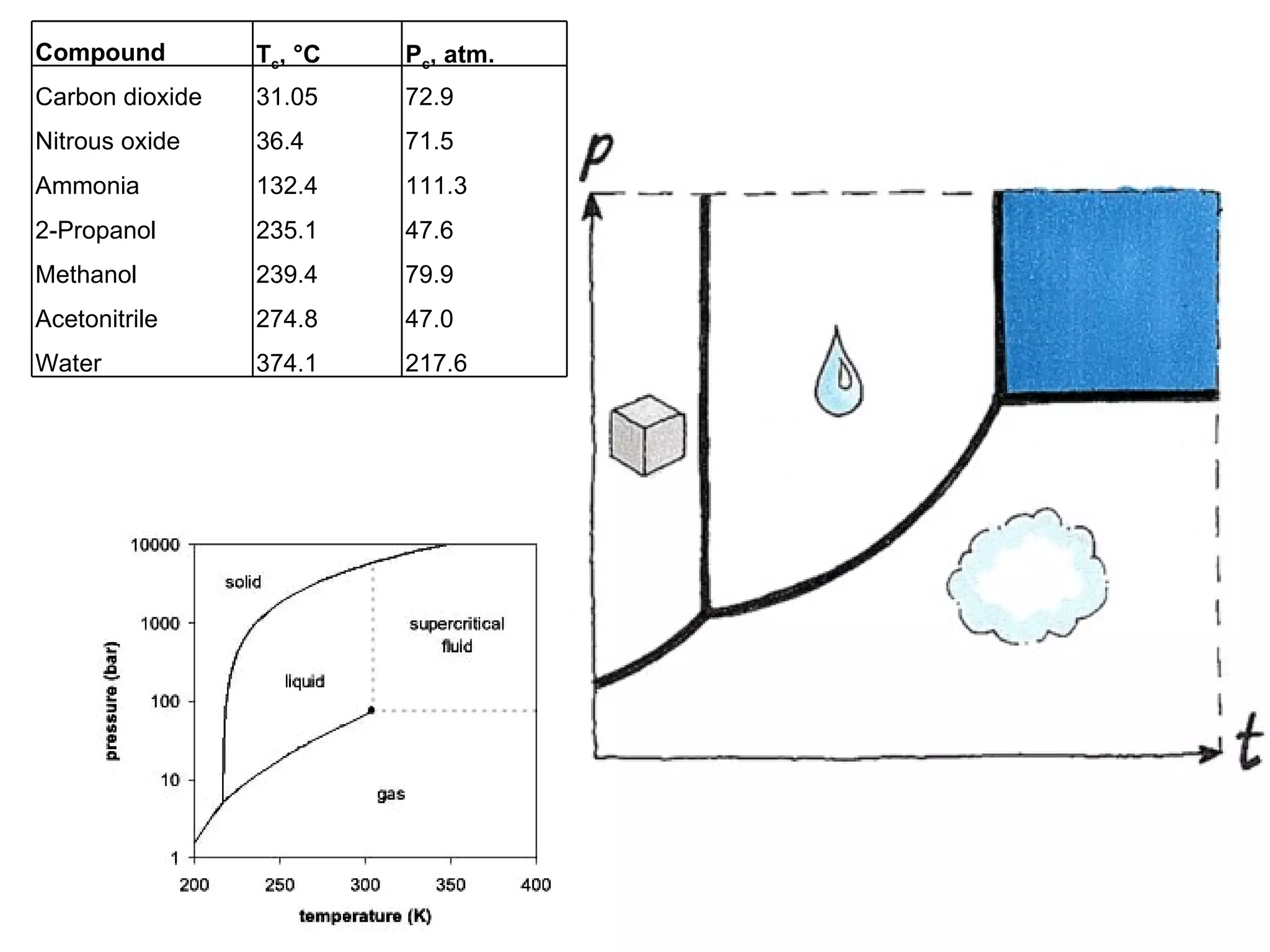
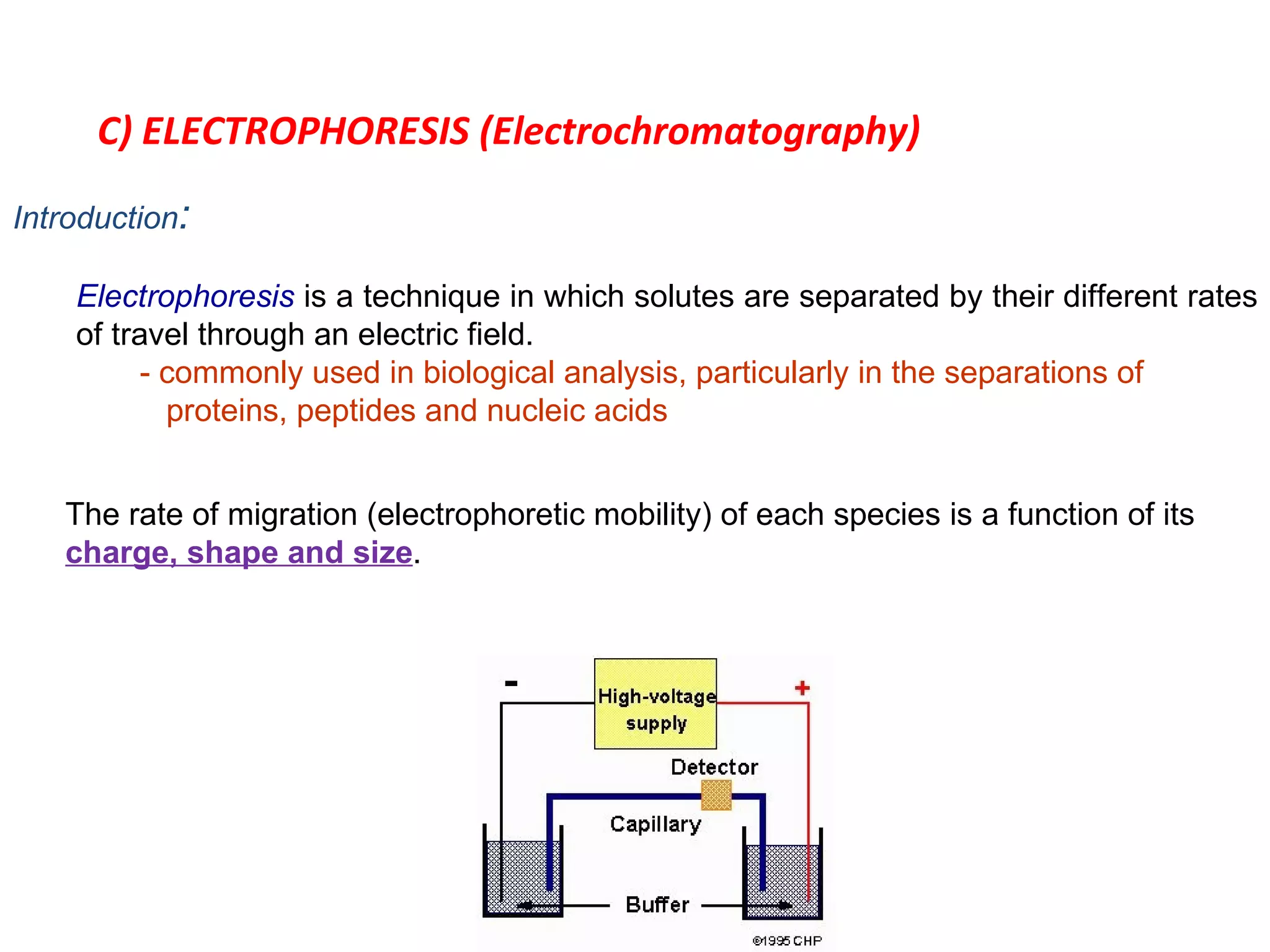
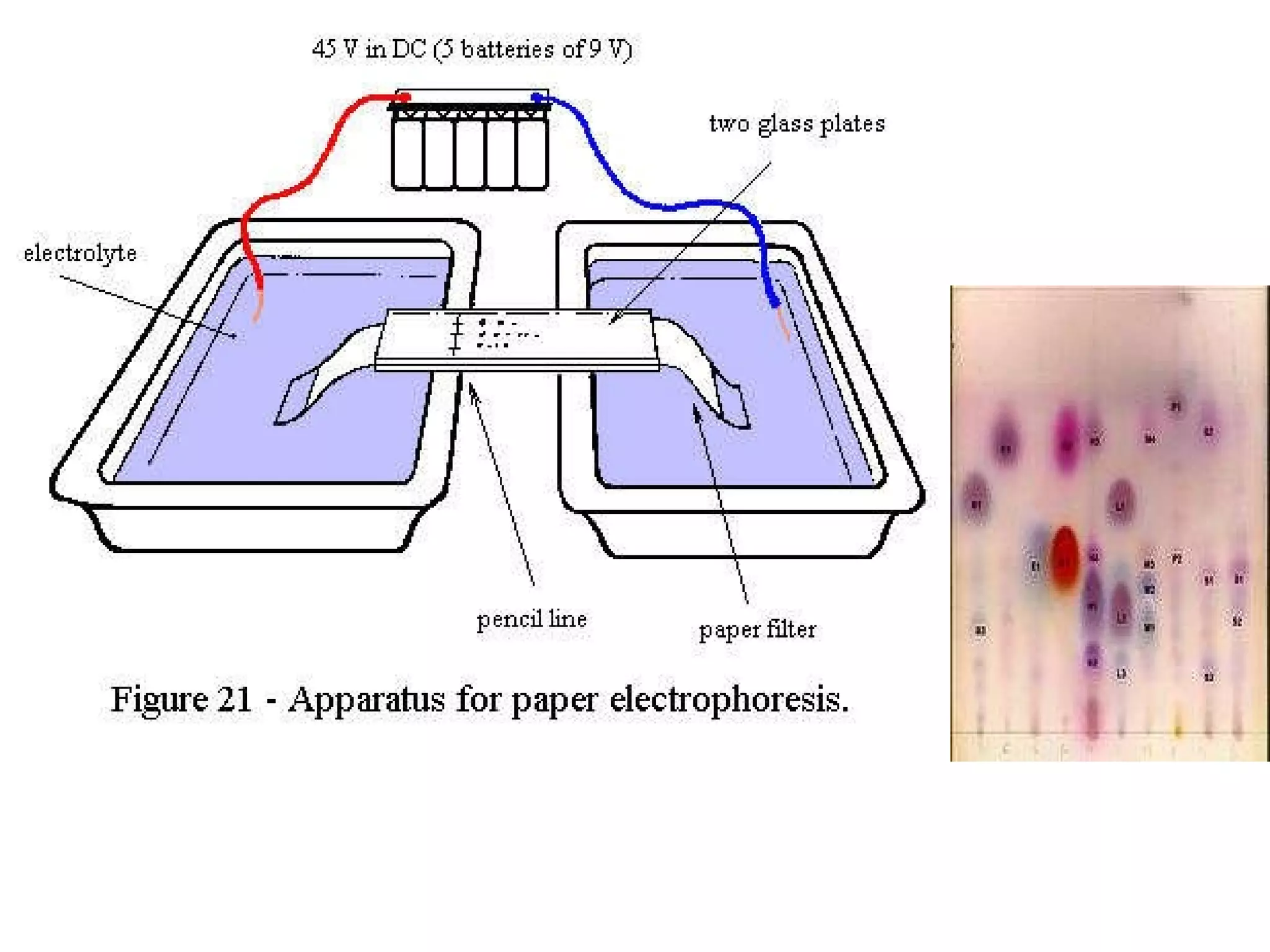
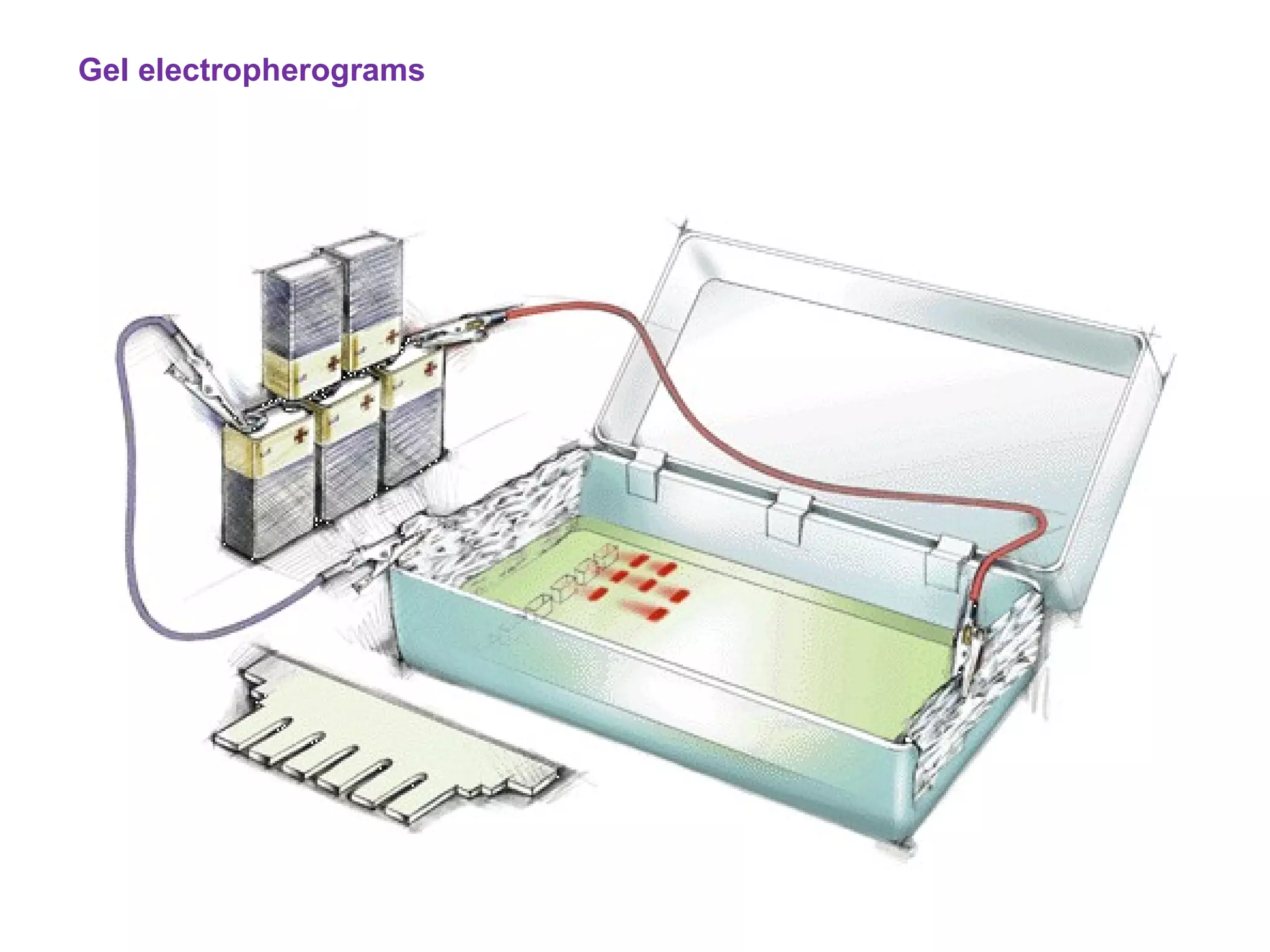
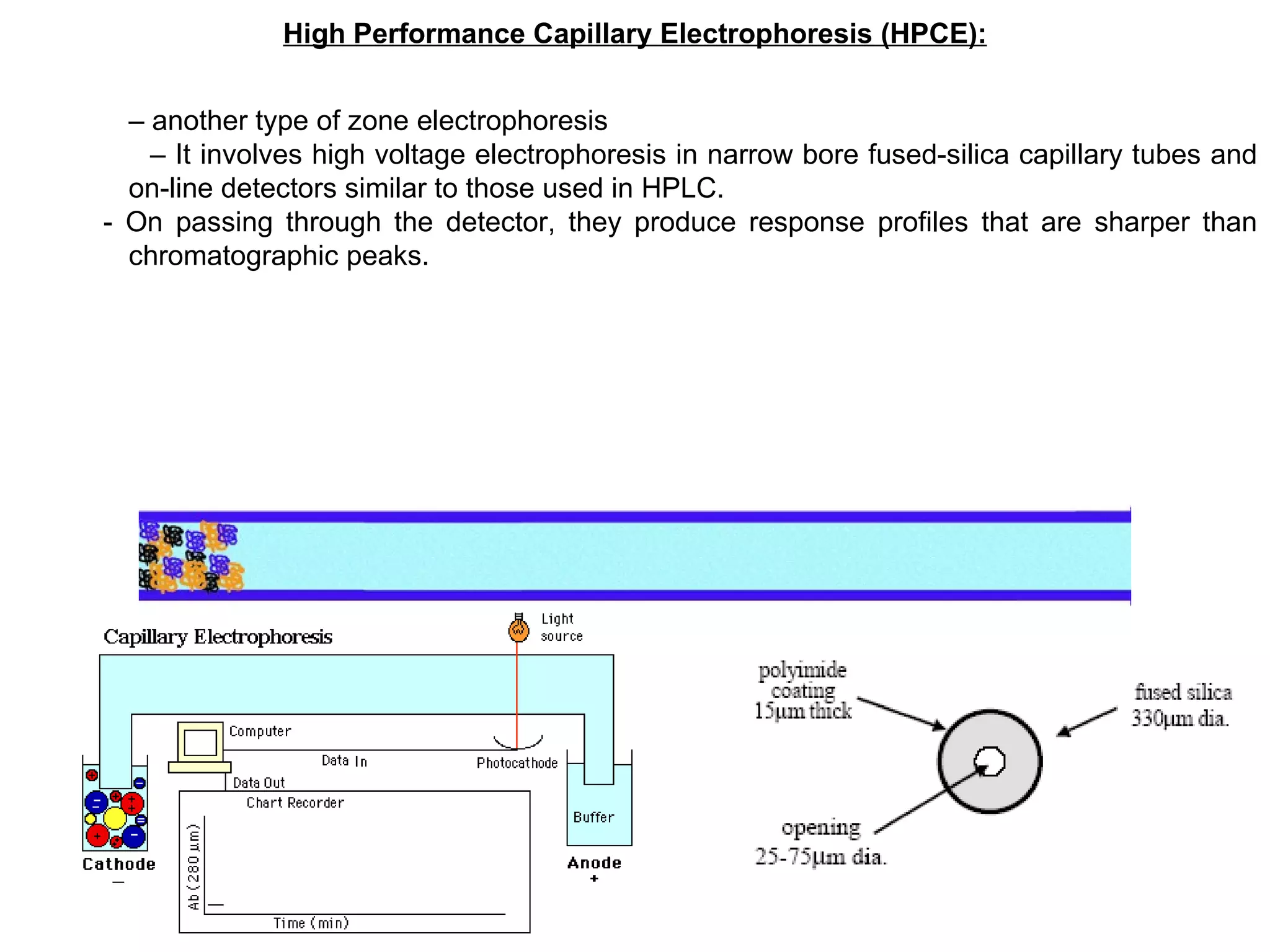
The document discusses various chromatography techniques including: open-column chromatography, paper chromatography, thin-layer chromatography, gas chromatography, high-pressure liquid chromatography, supercritical fluid chromatography, and electrophoresis. It provides details on the basic principles, instrumentation, applications and limitations of each technique.