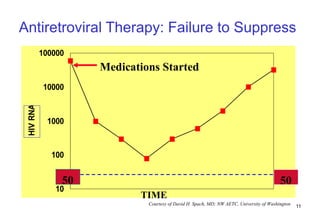
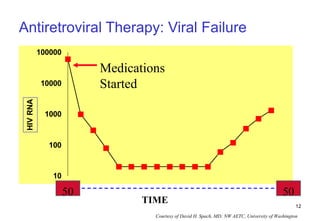
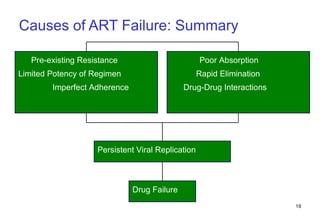
1. Mikael, a 28-year-old male with AIDS, was hospitalized for toxoplasmosis while on ART. A change in ART is recommended due to clinical treatment failure from a new opportunistic infection.
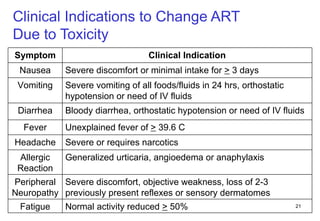
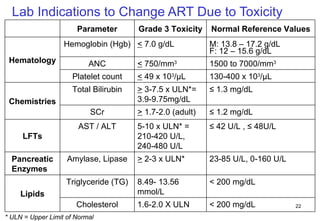
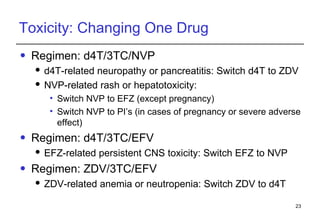
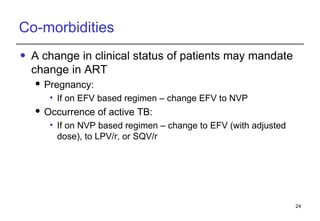
2. Factors to consider when changing ART include prior treatment history, resistance testing, side effects, adherence barriers, and monitoring procedures. Reasons for changing include treatment failure, toxicity, and co-morbidities like pregnancy or tuberculosis.
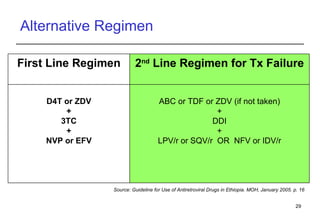
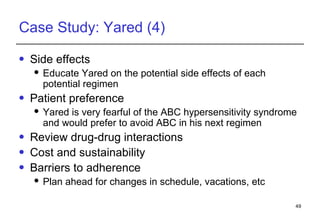
3. Yared, a 30-year-old man stable on ART for 4 years, shows immunologic and virologic treatment failure. Additional information is needed on his adherence and potential resistance before selecting an alternative regimen.